Abstract
Background
Whether hearing loss is independently associated with accelerated cognitive decline in older adults is unknown.
Methods
We studied 1984 older adults (mean age 77.4 years) enrolled in the HealthABC study, a prospective observational study begun in 1997–98. Our baseline cohort consisted of participants without prevalent cognitive impairment (Modified Mini-Mental State [3MS] scores ≥ 80) who underwent audiometric testing in Year 5. Participants were followed for 6 years. Hearing was defined at baseline using a pure-tone average (PTA) of thresholds at 0.5 – 4 kHz in the better-hearing ear. Cognitive testing was performed in Years 5, 8, 10, and 11 and consisted of the 3MS (measuring global function) and the Digit Symbol Substitution test (DSS, measuring executive function). Incident cognitive impairment was defined as a 3MS score < 80 or a decline in 3MS > 5 points from baseline. Mixed-effects regression and Cox models were adjusted for demographic and cardiovascular risk factors.
Results
Individuals with baseline hearing loss (PTA > 25 dB, n = 1162) had rates of decline in 3MS and DSS scores that were 41% and 32% greater, respectively, than those in normal hearing individuals (3MS: −0.65 points/year [95% CI: −0.73 – −0.56] vs. −0.46 points/year [95% CI: −0.55 – −0.36], p=.004; DSS: −0.83 points/year [95% CI: −0.94 – −0.73] vs. −0.63 points/year [95% CI: −0.75 – −0.51], p=.015). Compared to those with normal hearing, individuals with hearing loss had a 24% (Hazard ratio: 1.24 [95% CI: 1.05 – 1.48]) increased risk of incident cognitive impairment. Rates of cognitive decline and the risk of incident cognitive impairment were linearly associated with the severity of an individual’s baseline hearing loss.
Conclusion
Hearing loss is independently associated with accelerated cognitive decline and incident cognitive impairment in community-dwelling older adults. Further studies investigating the mechanistic basis of this association and whether hearing rehabilitative interventions could affect cognitive decline are needed.
The prevalence of dementia is projected to double every 20 years because of the aging of the world population1. Therefore, identifying factors and understanding mechanistic pathways that lead to cognitive decline and dementia in older adults is a public health priority. Some studies have suggested that hearing loss is independently associated with poorer cognitive functioning2–5 and incident dementia6, 7, possibly through the effects of hearing loss on cognitive load and/or mediation through reduced social engagement6. However, both cross-sectional8 and prospective studies9 have reported conflicting results that may be explained by variations in the study populations and the methods used for hearing and cognitive assessments.
Hearing loss is prevalent in nearly two-thirds of adults over 70 years and remains vastly undertreated10, 11. Determining if hearing loss is independently associated with cognitive decline is an important first step toward understanding whether the use of hearing rehabilitative interventions could help mitigate cognitive decline. In the present study, we investigate the association of hearing loss with cognitive trajectories and incident cognitive impairment over a 6-year period in a community-based, biracial cohort of older adults without prevalent cognitive impairment using standardized audiometric and cognitive tests.
Methods
Study population
Participants were enrolled in the Health, Aging and Body Composition (Health ABC) study, a prospective observational study that enrolled 3075 well-functioning, community-dwelling older adults aged 70–79 years from 1997–8 12, 13. Study participants were recruited from a random sample of white and black Medicare beneficiaries living within zip codes in Pittsburgh and Memphis that were within a one hour drive of the examination site. Only white and individuals were recruited because an original study objective was to examine race differences in body composition parameters, and there were insufficient resources to include other races or ethnicities. To be eligible, participants had to report no difficulty with walking a quarter mile, climbing 10 steps without resting, or performing basic activities of daily living.
Audiometric testing was administered in Year 5 (2001–2) of Health ABC. Of the 2206 participants who underwent hearing testing, 1984 had no evidence of cognitive impairment (defined by a Modified Mini-Mental State [3MS] ≥ 80), and these participants comprise our analytic (baseline) cohort. Various causes (e.g. attrition from death, drop out, missed study visit) prevented all participants enrolled at baseline (Year 1) from undergoing audiometric testing in Year 5. All study participants signed a written informed consent, and this study was approved by the institutional review boards of the study sites.
Audiometry
Audiometric assessments were performed in a sound-treated booth. Air-conduction thresholds in each ear were obtained from 0.25 to 8 kHz with TDH 39 headphones using a MA40 audiometer (Maico Diagnostics, Eden Prarie, MN) calibrated to American National Standards Institute standards (ANSI S3.6-1996). All thresholds were measured in decibels (dB) hearing level. A pure tone average (PTA) of hearing thresholds at 0.5–4 kHz was calculated for the better ear. Hearing loss was defined as a PTA > 25 dB per the World Health Organization’s definition of impairment 14 (the level at which hearing loss begins to impair daily communication).
Cognitive Assessments
The 3MS and the Digit Symbol Substitution (DSS) test were administered in Years 5 (2001–2), 8 (2004–5), 10 (2006–7), and 11 (2007–8). The 3MS is a global test with components for orientation, concentration, language, praxis, and memory 15. The maximum score is 100, and 3MS scores < 80 are considered indicative of cognitive impairment (a cutpoint of 80 is 91% sensitive and 97% specific for dementia) 15.The DSS is a non-verbal test of psychomotor speed and executive function16 in which participants code a series of numbers with the corresponding symbol in 90 seconds. We defined incident cognitive impairment as a 3MS score < 80 or a decline in 3MS > 5 points from baseline17–19.
Other Covariates
At enrollment, participants reported their age, sex, race, and educational history. Pre-specified algorithms based on both self-report and physician diagnoses, recorded medications, and laboratory data were used to define presence of hypertension (based on clinic measure, medications, or self-report) and diabetes mellitus (based on fasting blood glucose level, medications, or self-report). Stroke history, smoking status (current/former/never), and hearing aid use [“Do you wear a hearing aid?”] were based on interviewer-administered questionnaires. Risk factors for cognitive decline not known to be associated with hearing loss (e.g alcohol use, hyperlipidemia) were not included as covariates in the analytic model. In sensitivity analyses, participants were defined as remaining dementia-free if they did not use any dementia medications (memantine and acetylcholinesterase inhibitors) or have any dementia-related hospitalizations in review of hospitalization records 20. This limited diagnostic definition has been used previously20 and was only used in the present analyses in order to exclude potentially influential data points from patients with possibly more advanced dementia. Depressive symptoms at baseline were assessed with the 20-item Center for Epidemiologic Studies–Depression Scale (CES-D)21.
Statistical Analyses
Baseline characteristics of the study participants were compared with the Wilcoxon rank-sum test and chi-square. We created linear mixed effects (LME) models to assess the association of hearing loss with repeated measures of 3MS and DSS over time with individual-specific cognitive score and annual rate of change over time modeled as random effects. In LME models, an interaction term of hearing loss × time was included to assess whether hearing loss at baseline affected the individual rate of change in 3MS and DSS. Discrete-time Cox proportional hazard models were used to study time to incident cognitive impairment. All models were adjusted for demographic (age, sex, race, education, study site) and cardiovascular risk factors (smoking, hypertension, diabetes, and stroke history) as time-constant covariates unless otherwise specified. Regression model assumptions were checked with residual plots and histograms. Participants with missing covariate data (< 1% of the analytic cohort in all analyses) were excluded from analyses. Significance testing for all analyses was 2-sided with a type I error of 0.05. The statistical software used was SAS 9.2 (SAS Institute, Cary, North Carolina).
Results
At baseline, participants with hearing loss were more likely to be older, white, male, and have a positive smoking history than participants with normal hearing (Table 1). Individuals with hearing loss primarily had mild (PTA >25–40 dB, n = 762 [65.6%]) or moderate (PTA >40–70 dB, n = 386 [33.2%]) losses rather than a severe (>70 dB, n = 14 [1.2%]) hearing loss.
Table 1.
Demographic and clinical characteristics of baseline (Year 5) study cohort by hearing loss statusa
| Characteristic | Normal Hearing (n=822) |
Hearing Loss (n=1162) |
P |
|---|---|---|---|
| Age, mean (S.D.), y | 76.8 (2.7) | 77.9 (2.8) | <.001 |
| Race | |||
| Black | 345 (42.0) | 299 (25.7) | <.001 |
| White | 477 (58.0) | 863 (74.3) | |
| Male | 310 (37.7) | 641 (55.2) | <.001 |
| Education | |||
| < 12th grade | 132 (16.1) | 207 (17.8) | |
| High school graduate | 276 (33.7) | 399 (34.3) | .501 |
| Some college or greater | 411 (50.2) | 556 (47.9) | |
| Site | |||
| Memphis | 348 (42.3) | 563 (48.5) | .008 |
| Pittsburgh | 474 (57.7) | 599 (51.6) | |
| Smoking | |||
| Current | 43 (5.2) | 68 (5.9) | |
| Former | 369 (45.0) | 606 (52.4) | .002 |
| Never | 409 (49.8) | 483 (41.8) | |
| Hypertension | 639 (77.7) | 892 (76.8) | .625 |
| Diabetes | 140 (17.0) | 226 (19.5) | .177 |
| Stroke | 57 (6.9) | 111 (9.6) | .041 |
| Hearing aid use | 6 (0.7) | 251 (21.7) | <.001 |
| Pure tone average, mean (S.D.), dB | 18.1 (5.0) | 38.7 (10.7) | <.001 |
| CES-D, mean (S.D.)b | 4.70 (4.11) | 4.85 (4.28) | .426 |
Abbreviations: S.D. standard deviation
All values are expressed as No. (%) of participants unless otherwise indicated. Hearing loss is defined as a speech-frequency pure tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz in the better hearing ear of greater than 25 dB.
Centers for Epidemiologic Studies Depression Scale
In mixed-effects models adjusted for demographic and cardiovascular risk factors, hearing loss was associated with lower baseline 3MS scores (Table 2). On average, individuals with hearing loss had cognitive scores at baseline that were −0.75 points (95% CI: −1.17 – −0.33) lower for 3MS and −0.92 points (95% CI: −1.94 – 0.10) lower for DSS than scores in individuals with normal hearing. The association of other covariates with baseline cognitive scores are presented in eTable 1.
Table 2.
Adjusted mean baseline differences and annual rates of change in 3MS and DSS scores for individuals with normal hearing versus hearing lossa in multivariate mixed-effects modelsb
| Normal Hearing (n = 822) |
Hearing Loss (n = 1162) |
P | |
|---|---|---|---|
| 3MS Baseline score difference (95% CI) |
Reference | −0.75 (−1.17 – −0.33) |
<.001 |
| DSS Baseline score difference (95% CI) |
Reference | −0.92 (−1.94 – 0.10) |
.076 |
| 3MS Annual change (95% CI) |
−0.46 (−0.55 – −0.36) |
−0.65 (−0.73 – −0.56) |
.004 |
| DSS Annual change (95% CI) |
−0.63 (−0.75 – −0.51) |
−0.83 (−0.94 – −0.73) |
.015 |
Abbreviations: 3MS, modified Mini-Mental State Examination; DSS, Digit Symbol Substitution Test; CI, Confidence Interval
Hearing loss is defined as a speech-frequency pure tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz in the better hearing ear of greater than 25 dB.
All models are adjusted for age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke history.
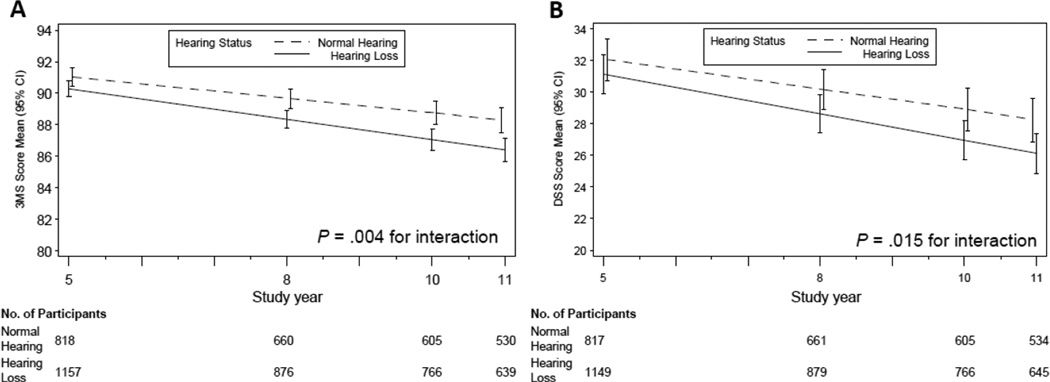
We investigated whether baseline hearing loss was associated with subsequent cognitive trajectories (Table 2). For 3MS, individuals with hearing loss had annual rates of decline that were 41% greater than for individuals with normal hearing (−0.65 points/year vs. −0.46 points/year, p = 0.004, difference of −0.19 points/year is 0.23 of the standard deviation of the estimated annual rates of decline in those with normal hearing [S.D. = 0.83]). On average, over the 6 year follow-up, we observed that individuals with hearing loss had adjusted 3MS scores that declined from 90.3 (95% CI:89.8 – 90.8) at baseline to 86.4 (95% CI: 85.7 – 87.1) at follow-up compared to 91.0 (95% CI: 90.5 – 91.6) at baseline and 88.3 (95% CI: 87.5 – 89.1) at follow-up for normal hearing individuals. For DSS, individuals with hearing loss had annual rates of decline that were 32% greater than individuals with normal hearing (−0.83 points/year vs. −0.63 points/year, p = 0.015, difference of −0.20 points/year is 0.33 of the standard deviation of the estimated annual rates of decline in those with normal hearing [S.D. = 0.60]). For DSS scores, individuals with hearing loss had scores of 31.1 (95% CI: 29.9 – 32.3) at baseline and 26.1 (95% CI: 24.8 – 27.4) at 6 year follow-up versus 32.0 (95% CI: 30.7 – 33.4) at baseline and 28.3 (95% CI: 26.9 – 29.6) at follow-up for individuals with normal hearing (Figure).
Figure. Multivariate mixed-effects models for adjusted mean (A) 3MS and (B) DSS scores by years of follow-up and hearing loss status.
3MS indicates Modified Mini-Mental State Examination. DSS indicates Digit Symbol Substitution test. Error bars indicate 95% confidence intervals. All models are adjusted for age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke history. The interaction term is between hearing loss and follow-up time.
Sensitivity analyses restricting the analytic cohort to those without severe hearing loss (n = 1970) or to those who remained dementia-free during follow-up (n = 1749) in order to exclude potentially influential data points did not substantively affect the results (cf Table 2). In this latter analysis of individuals remaining dementia-free, accelerated annual rates of cognitive decline were still observed in individuals with hearing loss compared to normal hearing (3MS: −0.46 points/year vs. −0.30 points/year, p = .002; DSS: −0.72 points/year vs. −0.54 points/year, p = .027). We also investigated whether adjustment for depressive symptoms as a possible mediator in the association of hearing loss with cognition would attenuate the observed association. In these analyses adjusted for CES-D scores at baseline, the magnitude of the association of hearing loss with accelerated cognitive decline was not substantively changed (cf Table 2).
We explored whether hearing loss severity at baseline was associated with the magnitude of the observed rate of subsequent cognitive decline. Compared to the rate of 3MS decline in normal hearing individuals (−0.45 points/year [95% CI: −0.55 – −0.36]), rates of 3MS decline in individuals with mild (−0.61 points/year [95% CI: −0.72 – −0.51], p =.031) or moderate or greater hearing loss (−0.71 points/year [95% CI: −0.85 – −0.56], p = .005) were significantly greater. Similarly, compared to the rate of decline in DSS scores in normal hearing individuals (−0.63 points/year [95% CI: −0.75 – −0.51]), rates of DSS decline in individuals with mild (−0.79 points/year [95% CI: −0.92 – −0.65], p =.090) or moderate or greater hearing loss (−0.92 points/year [95% CI: −1.11 – −0.74], p = .010) were also greater. Treating hearing loss as a continuous predictor variable yielded similar results. For 3MS and DSS, respectively, every 10dB of hearing loss at baseline was associated with an incremental additional rate of decline of −0.07 points/year (95% CI: −0.12 – −0.02, p = .003) and −0.06 points/year (95% CI: −0.12 – −0.004, p = .036).
We analyzed the association of baseline hearing loss with incident cognitive impairment in those individuals with at least one follow-up visit (n =1626) (Table 3). There were 609 cases of incident cognitive impairment during the 6 year follow-up. Individuals with hearing loss at baseline had a 24% increased risk of incident cognitive impairment during follow-up compared to normal hearing individuals (hazard ratio [HR] = 1.24, 95% CI: 1.05 – 1.48, p =.014). The magnitude of this association was linearly associated with the severity of an individual’s hearing loss at baseline (Table 3).
Table 3.
Cox proportional hazard models for incident cognitive impairmenta according to baseline hearing status (n = 1626b)
| Modelc | HR (95% CI) |
P |
|---|---|---|
| Hearing loss vs. Normal hearingd | 1.24 (1.05 – 1.48) |
.014 |
| Hearing loss category (reference normal hearing) | ||
| Mild hearing loss (PTA >25–40 dB) | 1.19 (0.99 – 1.44) |
.063 |
| Moderate or greater hearing loss (PTA >40 dB) | 1.36 (1.08 – 1.70) |
.008 |
| Per 10 dB of hearing loss | 1.07 (1.01 – 1.14) |
.030 |
Abbreviations: CI, confidence interval; HR: hazard ratio; 3MS, modified Mini-Mental State Examination; DSS, Digit Symbol Substitution Test
Cognitive impairment defined as 3MS score <80 or decline in 3MS score > 5 from baseline
The analytic cohort was restricted to individuals (n = 1626) with at least one follow-up visit after the baseline (Year 5) assessment
All models are adjusted for age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke history.
Hearing loss PTA > 25 dB; Normal hearing PTA ≤ 25 dB
We also examined whether hearing aid use among individuals with hearing loss was associated with cognitive trajectories. In these analyses restricted to individuals with moderate or greater hearing loss (individuals in whom hearing aid use was more common) and adjusted for demographic factors, individuals using hearing aids (n = 182) compared to those not using hearing aids (n=218) had higher baseline cognitive scores for 3MS (difference of 1.06 points [95% CI: 0.16 – 1.97], p = .021) but not for DSS (difference of 0.96 points [95% CI: −1.2 – 3.1], p = .383 ). Rates of cognitive decline were not significantly attenuated in individuals using hearing aids versus those without hearing aids (3MS: −0.62 points/year [95% CI: −0.84 – −0.41] vs. −0.77 points/year [95% CI: −0.98 – −0.56], p = .358; DSS: −0.82 points/year [95% CI: −1.06 – −0.58] vs. −0.98 points/year [95% CI: −1.22 – −0.75], p = .336). Hearing aid use was not significantly associated with a lower risk of incident cognitive impairment (HR: 0.82 [95% CI: 0.58 – 1.16], p = .256).
Comment
Our results demonstrate that hearing loss is independently associated with accelerated cognitive decline and incident cognitive impairment in community-dwelling older adults. The magnitude of these associations is clinically-significant with individuals with hearing loss having a 30–40% accelerated rate of cognitive decline and a 24% increased risk of incident cognitive impairment over a 6 year period compared to individuals with normal hearing. On average, individuals with hearing loss would require 7.7 years to decline by 5 points on the 3MS (a commonly accepted level of change indicative of cognitive impairment17–19) versus 10.9 years in those with normal hearing.
Our results are consistent with prior research demonstrating significant associations between greater hearing loss and poorer cognitive function on both verbal 3–5, 7, 8, 22–26 and non-verbal cognitive tests 2, 3, 5, 24, 26 and in both cross-sectional and prospective studies 5, 27. In contrast, other studies have not found similar associations 8, 9, 28.One key limitation across these prior studies is variability in how hearing loss was measured and how audiometric data were analyzed (e.g. choice of pure tone thresholds used to define hearing loss). Most studies utilized portable or screening audiometers 5, 8, 26, 28 or tested participants under varying environmental conditions (e.g. home-based testing) 26. The effect of biased or imprecise assessments of hearing thresholds would likely decrease sensitivity to detect associations due to increased variance. These prior studies have also generally been conducted in study populations from which the observed results may not be generalizable. Strengths of our current study are that our results are based on a population-based cohort of community-dwelling older adults, audiometric assessments of hearing using a definition of hearing loss adopted by the WHO 14, and both verbal and non-verbal cognitive tests.
A number of mechanisms may be theoretically implicated in the observed association between hearing loss and cognition. Poor verbal communication associated with hearing loss may confound cognitive testing, or vice-versa there may be an over-diagnosis of hearing loss in individuals with sub-clinical cognitive impairment. Miscommunication is unlikely given that hearing loss (short of severe-to-profound deafness) minimally impairs face-to-face communication in quiet environments (i.e. during cognitive testing) 29, particularly with testing administered by experienced examiners accustomed to working with older adults. Our results were also consistent using both verbal (3MS) and non-verbal (DSS) tests and were not sensitive to excluding individuals with severe hearing loss from the analytic cohort.
An over-diagnosis of hearing loss is also unlikely since there is no evidence that sub-clinical cognitive impairment would affect the reliability of audiometric testing. Behaviorally, pure-tone audiometry has been reliably performed in adults with early dementia 7 and is routinely performed in children as young as 4 years. There is also no evidence to suggest that older compared to younger adults adopt a more conservative response bias in reporting detection of the auditory signal during pure tone audiometry 30.
A shared neuropathologic etiology underlying both hearing loss and cognitive decline is a possibility but our study relied on a measure that primarily reflects peripheral hearing loss. Pure tone audiometry is typically considered a measure of the auditory periphery because detection of pure tones relies on cochlear transduction and neuronal afferents to brainstem nuclei without requiring significant higher auditory cortical processing31. Neuropathology associated with Alzheimer’s disease has not been found in the peripheral auditory pathways32, 33.
Finally, hearing loss may be mechanistically associated with cognitive decline, possibly through social isolation or cognitive load. Communication impairments caused by hearing loss can lead to social isolation and loneliness in older adults 34, 35, and epidemiologic 36, 37 and neuroanatomic studies 38 have demonstrated associations between loneliness with cognitive decline and dementia. The effect of hearing loss on cognitive load is suggested by studies demonstrating that under conditions where auditory perception is difficult (i.e. hearing loss), greater cognitive resources are dedicated to auditory perceptual processing to the detriment of other cognitive processes such as working memory 39–42. Neuroimaging studies have demonstrated a compensatory recruitment of regions in the prefrontal and temporoparietal cortex to maintain auditory speech processing in older adults 43, 44, and this pattern of neural compensation may explain the general preservation of language comprehension that is seen even in individuals with advanced dementia 45.
In the current study, hearing aid use was associated with slightly attenuated rates of cognitive decline and risk of cognitive impairment among individuals with hearing loss, but these results were not significant. Our study cohort may have been underpowered to detect a significant association, and data on other key variables (e.g. years of hearing aid use, adequacy of hearing aid fitting and rehabilitation, etc.) that would affect the success of hearing loss treatment and affect any observed association were not available. Contrary to popular perceptions, proper hearing rehabilitative treatment is complex, does not simply consist of using a hearing aid, and can vary substantially depending on the treating audiologist 46. These observational results also must be interpreted with caution because individuals choosing to use a hearing aid likely differ significantly from those individuals not using a hearing aid in both measured and unmeasured factors. Consequently, whether hearing rehabilitative strategies could affect cognitive decline remains unknown and will likely only be determined in a randomized controlled trial.
A key limitation of our study is that we cannot determine the mechanistic basis of the observed association between hearing loss and cognitive decline. In particular, hearing loss may plausibly contribute to an overall cycle of multimorbidity and frailty or synergistically interact with other known risk factors for dementia47–50, both of which could lead to cognitive decline in older adults. However, the hypothesized pathways underlying the association of hearing loss and cognition are not mutually exclusive, and hence, multiple pathways (e.g. shared neuropathology, cognitive load, increased loneliness) could likely co-exist and synergistically contribute to accelerated cognitive decline in individuals with hearing loss. Another limitation of our study is that hearing loss was only measured at baseline, and information was not available on the trajectory or the possible etiology of the hearing loss. However, it is unlikely that this limitation would lead to a differential bias in our results. Residual confounding by other environmental or neuropathologic processes is also plausible but speculative based on our current knowledge of known risk factors for hearing loss and cognitive decline.
Our results suggest that hearing loss is associated with accelerated cognitive decline and incident cognitive impairment in older adults. Further research investigating the mechanistic basis of this observed association and whether such pathways would be amenable to hearing rehabilitiative interventions is critically needed.
Acknowledgements
Funding/Support: This study was funded by National Institute on Aging (NIA) contracts N01-AG62101, N01-AG62103, N01-AG62106, NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH. Dr. Lin is supported in part by National Institute on Deafness and Other Communication Disorders grant K23DC011279 and a Triological Society/American College of Surgeons Clinician Scientist Award. Dr. Xue’s work was supported by the Johns Hopkins Older Americans Independence Center under Contract P30-AG02133 from the NIA.
Role of the Sponsor: The Intramural Research Program of the National Institute on Aging participated in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
Footnotes
Author contributions: Dr. Lin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lin, Simonsick
Acquisition of data: Yaffe, Harris, Purchase-Helzner, Satterfield, Ayonayon, Simonsick
Analysis and interpretation of data: Lin, Yaffe, Xia, Xue, Ayonayon, Ferrucci, Simonsick
Drafting of the manuscript: Lin
Critical revision of the manuscript for important intellectual content: Lin, Yaffe, Xia, Xue, Harris, Purchase-Helzner, Satterfield, Ayonayon, Ferrucci, Simonsick
Statistical analysis: Xia, Xue
Obtained funding: Lin
Administrative, technical, or material support: Lin, Yaffe, Harris, Purchase-Helzner, Satterfield, Ayonayon, Ferrucci, Simonsick
Study supervision: Lin, Simonsick
Conflict of Interest Disclosures: Dr. Lin reports being a consultant to Pfizer and an unpaid speaker for Cochlear Europe, a cochlear implant manufacturer.
Reference List
- 1.Alzheimer's Disease International. World Alzheimer Report. London: 2010. [Google Scholar]
- 2.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011 Nov;25(6):763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52(6):386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- 5.Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc. 2005 Mar;53(3):374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011 Feb;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916–1919. [PubMed] [Google Scholar]
- 8.Gussekloo J, de Craen AJ, Oduber C, van Boxtel MP, Westendorp RG. Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ Study. Am J Geriatr Psychiatry. 2005 Sep;13(9):781–786. doi: 10.1176/appi.ajgp.13.9.781. [DOI] [PubMed] [Google Scholar]
- 9.Gennis V, Garry PJ, Haaland KY, Yeo RA, Goodwin JS. Hearing and cognition in the elderly. New findings and a review of the literature. Arch.Intern.Med. 1991;151(11):2259–2264. [PubMed] [Google Scholar]
- 10.Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med. 2012 Feb 13;172(3):292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer ME, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, Tweed TS. Determinants of hearing aid acquisition in older adults. Am J Public Health. 2011 Aug;101(8):1449–1455. doi: 10.2105/AJPH.2010.300078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001 Oct;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 13.Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001 Jul;16(7):1343–1352. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. [Accessed 10/1/2011];Prevention of Deafness and Hearing Impaired Grades of Hearing Impairment. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/index.html.
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 16.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 17.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005 Jul;16(7):2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003 Jul 8;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 19.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003 Jan-Feb;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 20.Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011 Jan 19;305(3):261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff L. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Helzner EP, Cauley JA, Pratt SR, et al. Race and sex differences in age-related hearing loss: the Health, Aging and Body Composition Study. J.Am.Geriatr.Soc. 2005;53(12):2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 23.Ohta RJ, Carlin MF, Harmon BM. Auditory acuity and performance on the mental status questionnaire in the elderly. J Am Geriatr Soc. 1981 Oct;29(10):476–478. doi: 10.1111/j.1532-5415.1981.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 24.Granick S, Kleban MH, Weiss AD. Relationships between hearing loss and cognition in normally hearing aged persons. J Gerontol. 1976 Jul;31(4):434–440. doi: 10.1093/geronj/31.4.434. [DOI] [PubMed] [Google Scholar]
- 25.Thomas PD, Hunt WC, Garry PJ, Hood RB, Goodwin JM, Goodwin JS. Hearing acuity in a healthy elderly population: effects on emotional, cognitive, and social status. J Gerontol. 1983 May;38(3):321–325. doi: 10.1093/geronj/38.3.321. [DOI] [PubMed] [Google Scholar]
- 26.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994 Sep;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 27.Peters CA, Potter JF, Scholer SG. Hearing impairment as a predictor of cognitive decline in dementia. J.Am.Geriatr.Soc. 1988;36(11):981–986. doi: 10.1111/j.1532-5415.1988.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 28.Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001 Sep-Oct;47(5):289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- 29.Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J.Rehabil.Res.Dev. 2005;42(4 Suppl 2):9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- 30.Marshall L. Decision criteria for pure-tone detection used by two age groups of normal-hearing and hearing-impaired listeners. J Gerontol. 1991 Mar;46(2):P67–P70. doi: 10.1093/geronj/46.2.p67. [DOI] [PubMed] [Google Scholar]
- 31.Pickles JO. An introduction to the physiology of hearing. Bingley, UK: Emerald Group Publishing; 2008. [Google Scholar]
- 32.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer's disease. Neurology. 1993;43(4):779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- 33.Baloyannis SJ, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer's disease: a Golgi and electron microscope study. Acta Otolaryngol. 2009;129(4):416–418. doi: 10.1080/00016480802579074. [DOI] [PubMed] [Google Scholar]
- 34.Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40(3):320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J.Speech Hear.Res. 1982;25(4):593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- 36.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 37.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 39.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol.Aging. 2009;24(3):761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J.Acoust.Soc.Am. 1995;97(1):593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- 41.Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Q.J.Exp.Psychol. 1968;20(3):241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- 42.Rabbitt P. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol.Suppl. 1990;476:167–175. doi: 10.3109/00016489109127274. discussion 176.:167–175. [DOI] [PubMed] [Google Scholar]
- 43.Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006 Dec;96(6):2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- 44.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011 Aug 31;31(35):12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rousseaux M, Seve A, Vallet M, Pasquier F, Mackowiak-Cordoliani MA. An analysis of communication in conversation in patients with dementia. Neuropsychologia. Nov;48(13):3884–3890. doi: 10.1016/j.neuropsychologia.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Lin FR. Hearing loss in older adults: who's listening? JAMA. 2012 Mar 21;307(11):1147–1148. doi: 10.1001/jama.2012.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010 Aug 3;153(3):176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 48.Daviglus ML, Plassman BL, Pirzada A, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011 Sep;68(9):1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 49.Plassman BL, Williams JW, Jr., Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010 Aug 3;153(3):182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 50.Yaffe K, Fiocco AJ, Lindquist K, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009 Jun 9;72(23):2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]