Table 4.
One-pot MW-assisted synthesis of a set of urea derivatives.a
 | ||||
| entry | R–Br | R–NH2 | product | yieldb (%) |
| 1 |
 17 |
 22 |
 27 |
98 |
| 2 | 17 |
 23 |
 28 |
98 |
| 3 | 17 |
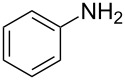 24 |
 29 |
98 |
| 4 |
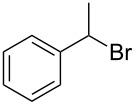 18 |
22 |
 10 |
97 |
| 5 | 18 | 23 |
 30 |
94 |
| 6 | 18 | 24 |
 31 |
98 |
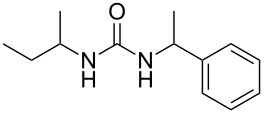
| 7 | 18 |
 25 |
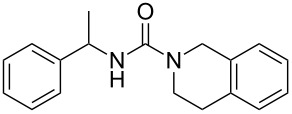 32 |
98 |
| 8 | 18 |
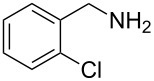 26 |
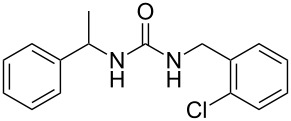 33 |
98 |
| 9 |
 19 |
22 |
 34 |
89 |
| 10 | 19 | 23 |
 35 |
89 |
| 11 | 19 | 24 |
 36 |
88 |
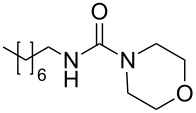
| 12c |
 20 |
3 |
 37 |
85 |
| 13c |
 21 |
3 |
 38 |
83 |
aReactions were carried out in a MW reactor: alkyl bromide, NaN3 (2 equiv), MeCN, 95 °C, 3 h ; then PS-PPh2 (1.5 equiv), CO2 (14.5 bar), amine (2 equiv), at 50 °C 1.5 h then 70 °C 3 h. bIsolated yield. cR–N3 was synthesized in DMF and MeCN was then added.
