Table 1.
Optimization of conditions for CM of 10 and methyl vinyl ketone (8).a
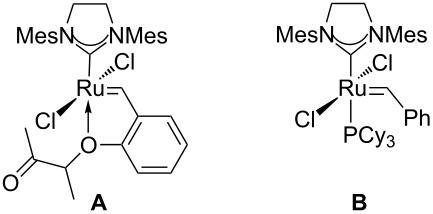 | ||||
| entry | catalyst (mol %) | solvent | T | yield of 11 |
| 1 | A (2.0) | CH2Cl2 | 40 °C | 76% |
| 2b | A (5.0) | CH2Cl2 | 40 °C | 51% |
| 3 | A (0.5) | CH2Cl2 | 40 °C | 67% |
| 4 | A (1.0) | CH2Cl2 | 40 °C | 85% |
| 5 | B (2.0) | toluene | 80 °C | 61% |
| 6c | B (2.0) | toluene | 80 °C | 78% |
| 7 | B (5.0) | CH2Cl2 | 40 °C | 93% |
aGeneral conditions: 8.0 equiv of 8, initial substrate concentration: c = 0.5 M; bformation of (E)-hex-3-ene-2,5-dione observed in the 1H NMR spectrum of the crude reaction mixture. cWith phenol (0.5 equiv) as additive.
