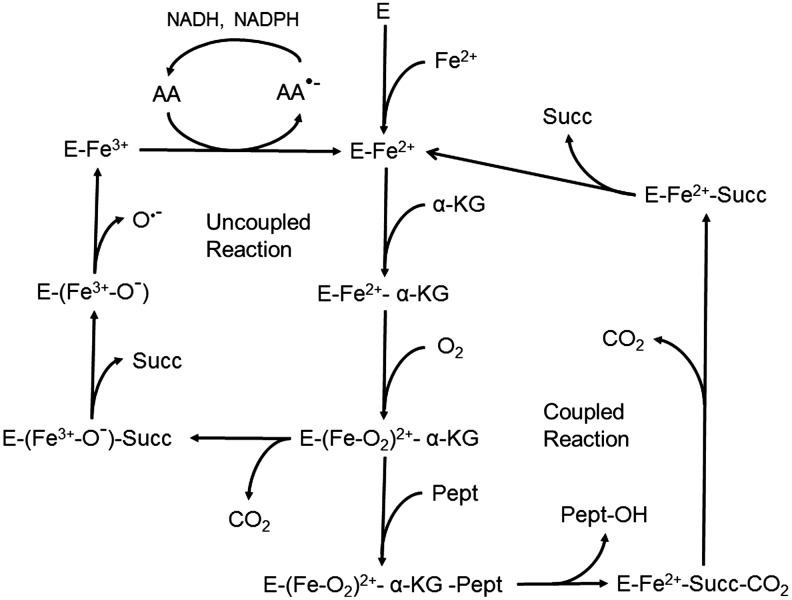
FIG. 7.
Role of ascorbate in maintaining the activity of α-ketoglutarate-dependent hydroxylation reactions. The proposed mechanism of α-ketoglutarate-dependent hydroxylases is first a sequential binding of ferrous iron, α-ketoglutarate, and molecular oxygen. At this point, there is the formation of a key ferryl-oxygen intermediate that not only usually binds the substrate protein (Pept), but can also generate an uncoupled cycle by cleaving α-ketoglutarate to succinate (Succ) and releasing CO2. In the functional enzyme cycle, a proline or lysine is hydroxylated with subsequent sequential release of the modified protein, CO2, and succinate leaving enzyme-bound ferrous iron that can restart the cycle. In an uncoupled cycle, the enzyme-bound iron is oxidized to ferric iron by oxygen, and the latter is released, perhaps as the hydroxyl radical (O•−). The function of ascorbate is to reduce the enzyme-bound ferric to ferrous iron, thus allowing the enzyme to enter a potentially functional cycle again.