Summary
The arenavirus Lassa virus (LASV) causes a severe haemorrhagic fever with high mortality in man. The cellular receptor for LASV is dystroglycan (DG). DG is a ubiquitous receptor for extracellular matrix (ECM) proteins, which cooperates with β1 integrins to control cell–matrix interactions. Here, we investigated whether LASV binding to DG triggers signal transduction, mimicking the natural ligands. Engagement of DG by LASV resulted in the recruitment of the adaptor protein Grb2 and the protein kinase MEK1 by the cytoplasmic domain of DG without activating the MEK/ERK pathway, indicating assembly of an inactive signalling complex. LASV binding to cells however affected the activation of the MEK/ERK pathway via α6β1 integrins. The virus-induced perturbation of α6β1 integrin signalling critically depended on high-affinity LASV binding to DG and DG’s cytoplasmic domain, indicating that LASV–receptor binding perturbed signalling cross-talk between DG and β1 integrins.
Introduction
Lassa virus (LASV) is the causative agent of a severe haemorrhagic fever with high mortality in humans that is endemic to West Africa and infects several hundred thousand individuals per year with thousands of deaths (Geisbert and Jahrling, 2004). There is neither a licensed vaccine nor an efficacious treatment for this disease, resulting in 15–30% mortality in hospitalized patients (McCormick and Fisher-Hoch, 2002). Despite the widespread viral replication in fatal Lassa fever cases, histological analysis revealed only modest infiltration of inflammatory cells (Walker et al., 1982). The terminal shock syndrome associated with fatal Lassa fever occurs without evidence of massive cellular necrosis, vascular damage or bleeding, suggesting that direct perturbation of host cell function by LASV contributes to disease (McCormick and Fisher-Hoch, 2002; Kunz, 2009; Moraz and Kunz, 2011). The analysis of the virus–host cell interaction and the consequences for host cell function are therefore important to understand the viral pathogenesis underlying fatal Lassa fever.
LASV belongs to the family Arenaviridae, enveloped negative strand RNA viruses with a non-lytic life cycle confined to the cytoplasm (Buchmeier et al., 2007). The bisegmented genome of LASV codes for only four proteins, an RNA-dependent RNA polymerase (L), the viral nucleoprotein (NP), the matrix protein (Z) and the precursor of the viral envelope glycoprotein (GPC) (Buchmeier et al., 2007). The GPC precursor is processed by the cellular protease subtilisin kexin isozyme-1(SKI-I)/site-1 protease (S1P) yielding the mature envelope proteins GP1 and GP2. (Lenz et al., 2001; Beyer et al., 2003; Rojek et al., 2008c). The GP1 part is implicated in binding to cellular receptor(s), whereas GP2 resembles fusion-active portions of other viral GPs (Eschli et al., 2006; Igonet et al., 2011).
The first cellular receptor discovered for LASV and other Old World arenaviruses is α-dystroglycan (α-DG), the peripheral subunit of DG, a ubiquitously expressed versatile receptor for extracellular matrix (ECM) proteins (Cao et al., 1998; Oldstone and Campbell, 2011). Very recently, additional candidate receptors for LASV have been reported: the C-type lectins DC-SIGN, LSECtin, as well as the Tyro3/Axl/Mer (TAM) receptor tyrosine kinases Axl and Tyro3 (Shimojima et al., 2012). While the C-type lectins have expression patterns limited to specific cell types, the TAM receptors Tyro3 and in particular Axl are widely expressed, including cell types positive for DG. Notably, in cells coexpressing DG and Axl, DG seems to be the preferred receptor for LASV (Shimojima et al., 2012), suggesting a role for TAM receptor kinases as LASV receptors primarily in cells lacking functional DG.
Dystroglycan is expressed in most developing and adult tissues, typically in cell types that adjoin basement membranes (Durbeej et al., 1998) and is crucial for normal cell–matrix adhesion (Henry and Campbell, 1998; Henry et al., 2001). Mutations affecting DG in humans result in severe congenital diseases (Barresi and Campbell, 2006). Encoded as a single protein, DG is proteolytically processed into α-DG, which is extracellular, and β-DG which is membrane anchored (Barresi and Campbell, 2006). DG provides a molecular link between the ECM and the actin-based cytoskeleton. Alpha-DG binds with high affinity to the ECM proteins laminin, agrin, perlecan and neurexins and is non-covalently associated with β-DG that interacts with the cytoskeletal adaptor proteins dystrophin and utrophin, which anchor the DG complex to the actin-based cytoskeleton (Ervasti and Campbell, 1991; 1993). The cytoplasmic domain of β-DG was found to interact with the signalling adaptor molecule Grb2 (Yang et al., 1995), the protein kinases MEK, ERK and focal adhesion kinase (FAK) (Yang et al., 1995; Cavaldesi et al., 1999; Spence et al., 2004), suggesting a role of DG in cellular signal transduction.
Several lines of evidence support a functional interaction between DG and β1 integrins in the assembly of laminin-based ECM structures (Henry et al., 2001). Laminins recognize α-DG and β1 integrins via distinct LG modules located in the C-terminal G domain of their α-chain, LG4 and LG1–3 respectively (Hohenester et al., 1999). Interestingly, DG and α6β1 integrins seem to have opposing roles in the regulation of the canonical MEK/ ERK MAP kinase pathway in response to laminin (Ferletta et al., 2003). Binding of laminin LG1–3 to α6β1 integrins activates MEK/ERK signalling, whereas attachment of LG4 to α-DG resulted in a dose-dependent reduction of MEK/ERK activation (Ferletta et al., 2003). Simultaneous binding of laminin to the two receptors likely results in a defined equilibrium of MEK/ERK signalling critical for normal cell–matrix adhesion.
In mammals, α-DG undergoes complex post-translational modifications that are crucial for its function as an ECM receptor (Barresi and Campbell, 2006; Kanagawa and Toda, 2006). Of particular importance are specific O-glycan modifications of α-DG’s mucin-type domain, which involve protein O-mannosylation and modifications by the glycosyltransferases LARGE and LARGE2 (Barresi et al., 2004). We and others have shown that protein O-mannosylation and LARGE-dependent modifications are also crucial for α-DG’s function as a high-affinity receptor for arenaviruses (Kunz et al., 2005b) and that the viruses closely mimic the molecular mechanisms of receptor recognition by ECM proteins (Rojek et al., 2007a). As a consequence LASV competes with ECM proteins for receptor binding, resulting in displacement of DG-bound ECM (Kunz et al., 2005a), likely affecting DG’s function in the host cell. In the present study, we investigated the impact of LASV binding to α-DG on DG-mediated signal transduction. We found that engagement of LASV affected the interaction of DG with signalling molecules and perturbed the signalling cross-talk with β1 integrins.
Results
Binding of inactivated LASV to cellular DG triggers recruitment of Grb2 and MEK1 without activating MEK1
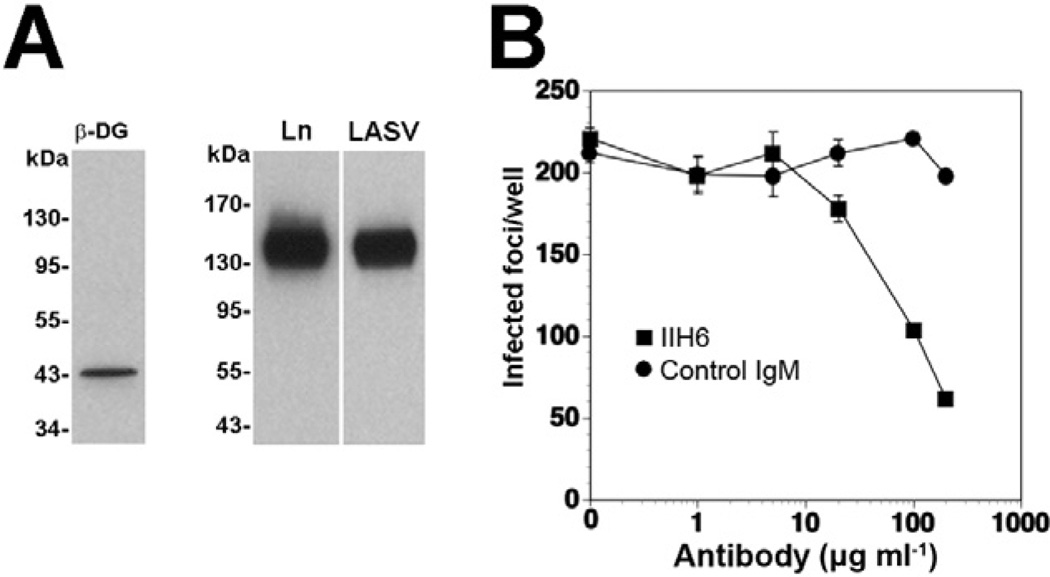
Considering the high binding affinity of LASV for α-DG and the multivalent nature of the virus particles, we hypothesized that virus attachment may result in extensive receptor clustering with possible effects on DG-mediated signal transduction. Since LASV is a BSL4 pathogen, work with live virus is restricted to laboratories with high security containment. To circumvent this problem, we used either inactivated virus or recombinant retroviruses bearing the envelope glycoprotein of LASV (Reignier et al., 2006; Rojek et al., 2006). Since receptor binding and host cell entry of arenaviruses are mediated exclusively by the viral GP, recombinant retroviruses displaying LASV GP adopt the receptor binding characteristics of LASV (Reignier et al., 2006). They represent a suitable BSL2 surrogate for LASV and have been widely used to study LASV–receptor interactions (Kunz et al., 2005a,b; Reignier et al., 2006; Rojek et al., 2007a). As a cell culture model, we chose the human lung epithelial cell line WI-26 VA4, which had previously been utilized for studies on DG signalling (Ferletta et al., 2003). Alpha-DG isolated from WI-26 VA4 cells by lectin affinity purification bound inactivated LASV with high affinity, as assessed by virus overlay protein binding assay (Fig. 1A). In a next step, we verified if cell attachment and entry of LASV into WI-26 VA4 cells was indeed mediated by α-DG. For this purpose, cells were pre-treated with increasing concentration of the monoclonal antibody (mAb) IIH6 that recognizes a functional glycan epitope on α-DG (Kanagawa et al., 2004) and competes with virus binding (Kunz et al., 2005b). Cells were then infected with LASV pseudotypes containing a green fluorescent protein (GFP) reporter in their genome at a multiplicity of infection (moi) of 0.01 infectious virus particle per cell. Infected cells were detected after 24 h by immunofluorescence (IF) detection of the GFP reporter. Pre-treatment with mAb IIH6, but not an IgM isotype control significantly blocked infection with LASV pseudotypes in a dose-dependent manner, confirming DG as a major receptor for LASV in these cells (Fig. 1B). The only partial inhibition of LASV pseudotype infection in presence of 200 µg ml−1 mAb IIH6 is consistent with earlier studies (Kunz etal., 2005b) and is likely due to the high binding affinity of LASV GP to α-DG.
Fig. 1. Cell entry of LASV pseudotypes in WI-26 VA4 human lung epithelial cells is mediated by DG.
A. WI-26 VA4 cells express DG that is functional as LASV receptor. WI-26 VA4 cells were lysed and glycoproteins enriched by affinity purification with the lectin wheat germ agglutinin. Functional α-DG was detected by laminin overlay assay (Ln) and virus overlay protein binding assay using inactivated virus (LASV).
B. Blocking of infection with mAb IIH6. Monolayers of WI-26 VA4 cells were blocked with mAb IIH6 or an unrelated mouse IgM (Control IgM) at the indicated concentrations for 2 h at 4°C. Next, LASV pseudotypes (200 PFU) were added for 45 min. Infection was assessed after 24 h by immunofluorescence staining for GFP. Infected foci were counted in each well (means ± SD, n = 3).
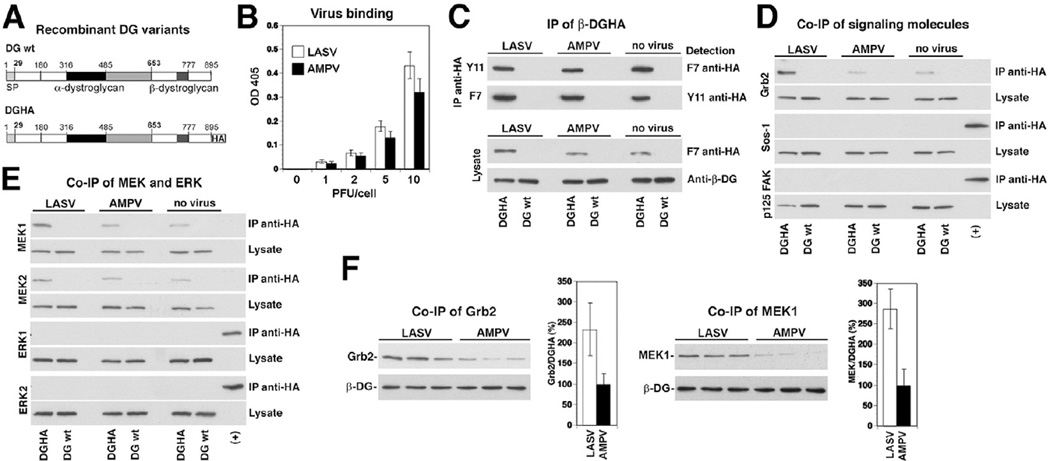
The cytoplasmic tail of β-DG can associate with signalling molecules, including Grb2, MEK, ERK and FAK, suggesting a role in cellular signal transduction (Yang etal., 1995; Cavaldesi etal., 1999; Spence etal., 2004). We next assessed the impact of LASV binding to cellular α-DG on the association of β-DG with these signalling molecules. Since available anti-β-DG antibodies recognize the cytoplasmic domain, interference with the binding of signalling molecules was a concern. To circumvent this problem, we made use of a recombinant full-length DG containing a C-terminal spacer sequence of four amino acids (GGGS), followed by an HA-tag (DGHA) (Fig. 2A). Previous studies demonstrated that this C-terminal tagging of β-DG had no influence on the biosynthesis, transport and function of DG (Rojek etal., 2007b). Considering the biosafety restrictions of work with live LASV and to avoid any possible effect due to virus replication, we used inactivated LASV to study the effect of virus binding on DG signalling. For this purpose, LASV (strain Josiah) was grown in a BSL4 facility of the Centers for Disease Control and inactivated by gamma irradiation as described (Spiropoulou et al., 2002). As a control, we used inactivated Amapari virus (AMPV), a New World arenavirus that does not use DG as a receptor (Spiropoulou et al., 2002). Previous studies had shown that our inactivation protocol abrogated virus replication, but did not affect receptor binding (Spiropoulou et al., 2002). In a first step, we determined binding of LASV and AMPV to WI-26 VA4 cells. Briefly, monolayers of WI-26 VA4 cells were cultured in microtitre plates, chilled on ice and incubated with increasing concentrations of inactivated virus. After removing unbound virus, cells were fixed. Bound virus was detected with mAb 83.6 that recognizes a highly conserved epitope in LASV and AMPV GP2 (Weber and Buchmeier, 1988), combined with a HRP-labelled secondary antibody in a colour reaction. Both LASV and MAPV bound WI-26 VA4 cells to a similar extent in a dose-dependent manner (Fig. 2B). Next, WI-26 VA4 cells were transfected with DGHA, using un-tagged DG as a control. After 48 h, cells were incubated with inactivated LASV and AMPV (100 particles per cell) in the cold, allowing virus attachment without receptor clustering. After removal of unbound virus, the temperature was shifted to 37°C for 10 min. Cells were rapidly chilled, cold detergent extracts prepared and cleared lysates subjected to immunoprecipitation (IP) with anti-HA matrix. Immunocomplexes were probed for the presence of DGHA, Grb2, Sos, FAK, MEK1/2 and ERK1/2 in Western blot. Similar amounts of DGHA were detected in IPs from cells incubated with LASV, AMPV and mock-treated cells (Fig. 2C), excluding receptor downregulation and/or degradation as a consequence of virus binding. In line with published data (Yang et al., 1995; Spence et al., 2004), we detected an association of β-DG with Grb2 and MEK1/2, whereas neither FAK, Sos nor ERK was detected in anti-HA immunocomplexes in this cell type under our experimental conditions (Fig. 2D and E). Binding of LASV, but not AMPV resulted in a significant increase in the amounts of β-DG-associated Grb2 (Fig. 2D and F) and MEK1 (Fig. 2E and F), indicating recruitment of Grb2 and MEK1 upon virus binding. In a next step, we investigated if the observed LASV-induced recruitment of Grb2 and MEK1 resulted in activation of MEK1. For this purpose, we exposed cells to LASV and AMPV, isolated HA-tagged β-DG at different time points as described above. Immunocomplexes were probed with a mAb specific for phosphorylated (activated) MEK1 in Western blot and signals normalized to total MEK1. At no time point did we observe significant phosphorylation of MEK1 (data not shown), suggesting that the observed virus-induced recruitment of Grb2 and MEK1 results in assembly of an inactive signalling complex associated with β-DG.
Fig. 2. Binding of inactivated LASV to cells promotes association of β-DG with Grb2.
A. Schematic representation of C-terminally tagged DG (DGHA). The N-terminal domain (white), the mucin-type domain (black) and the C-terminal domain (grey) of α-DG, β-DG and the C-terminal HA tag are indicated.
B. Binding of inactivated LASV and AMPV to WI-26 VA4 cells: cell monolayers were incubated with the indicated concentrations of inactivated virus in the cold. Bound virus was detected with mAb 83.6 to arenavirus GP2, combined with a HRP-conjugated secondary antibody in a colour reaction (means ± SD, n = 3).
C. IP of β-DGHA: WI-26 VA4 cells transiently transfected with either DGHA or wild-type DG (DG wt) were seeded on poly-l-lysine and incubated with inactivated LASV, AMPV, or no virus at a particle per cell ratio of 100. After 20 min, cells were lysed and DGHA precipitated with either a polyclonal rabbit antibody anti-HA Y11 or mouse mAb F7 anti-HA. Immunocomplexes were probed for HA in Western blot using the indicated antibodies. Total-cell lysates were probed for DGHA with mAb F7 anti-HA and for β-DG with pAb AP83 anti-β-DG.
D and E. Co-immunoprecipitation (co-IP) of β-DGHA with signalling molecules: immunocomplexes and total lysates (C) were probed for the presence of Grb2, Sos-1, FAK, MEK1/2 and ERK1/2 in Western blot. In case of Sos-1, FAK and ERK1/2, a positive control corresponding to 0.1% of total-cell protein was included (+).
F. Binding of inactivated LASV increases the association of β-DG with Grb2 and MEK1: triplicate specimens of WI-26 VA4 cells transiently transfected with DGHA were exposed to inactivated viruses, lysed and DGHA precipitated as in (C). DGHA, Grb2 and MEK1 were detected in Western blot as in (D) and (E). For quantitative analysis, X-ray films were scanned with a densitometer and the ratios of Grb2/DGHA and MEK1/Grb2 calculated. For normalization, signals obtained with the AMPV-negative control were defined as 100% (n = 3, ±SD).
Infection with LASV pseudotypes depends on DG, but not β1 integrins
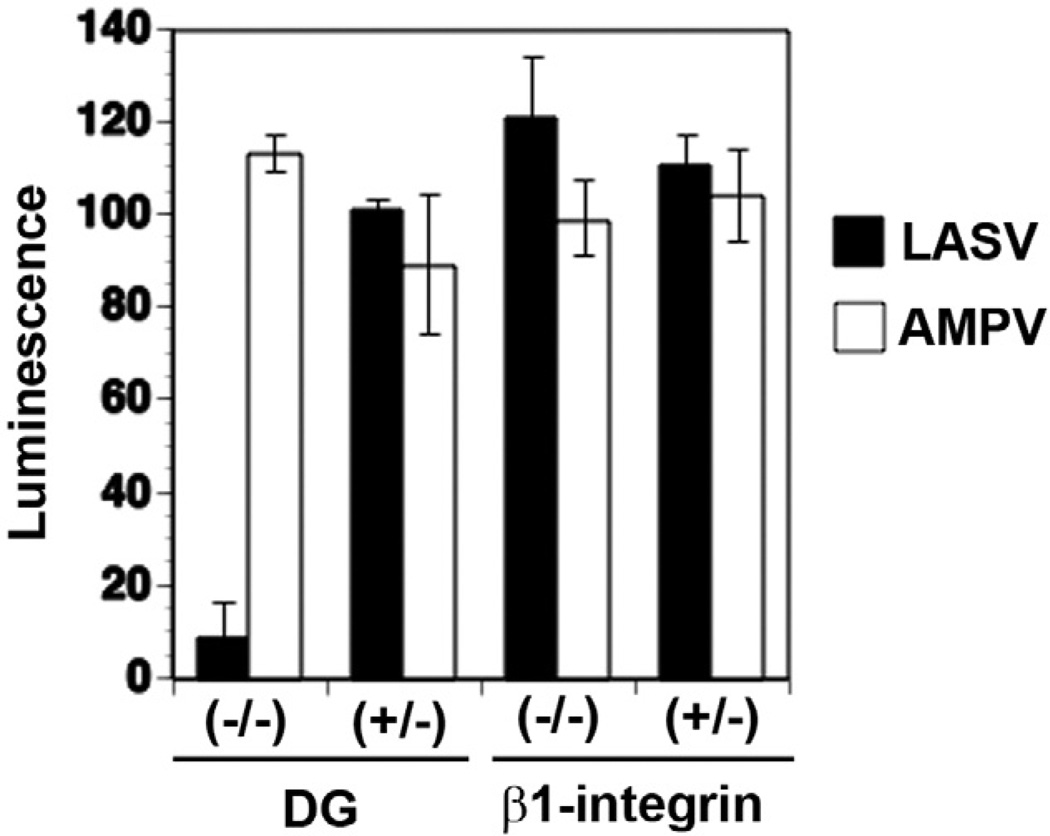
Several lines of evidence indicate that DG functionally interacts with β1 integrins in the host cell (Henry etal., 2001; Ferletta etal., 2003). While the role of DG in host cell attachment and entry of LASV has been well established, a possible function of β1 integrins in LASV cell entry had not yet been addressed. To address this issue, we exploited the fact that LASV efficiently infects wild-type murine embryonic stem (ES) cells (Spiropoulou etal., 2002). To define a possible role of β1 integrins in LASV cell entry, we employed a murine ES cell line deficient in β1 integrin (−/−) and the parental hemizygous (+/−) line (Fassler and Meyer, 1995; Fassler etal., 1995). To separate virus entry mediated by the envelope GP of LASV from subsequent steps of virus replication, we employed recombinant retroviruses pseudotyped with LASVGP that contained a luciferase reporter in their genome (Rojek etal., 2007a). As a control, we used retroviral pseudotypes containing the GP of AMPV, which is independent of DG. Murine ES cell with the genotypes DG (+/−), DG (−/−), β1 integrin (+/−) and β1 integrin (−/−) were infected with retroviral pseudotypes bearing the GPs of LASV and AMPV. Since murine retroviruses show relatively low levels of reporter gene expression in murine ES cells (Reignier etal., 2006), pseudotype infection was performed at high moi (10). Infection was assessed after 48 h by luciferase assay. In line with previous reports, cells deficient in DG showed markedly reduced susceptibility to LASV pseudotypes, whereas AMPV pseudotype infection was not affected (Fig. 3). In contrast, cells deficient in β1 integrins showed similar susceptibility to both pseudotypes (Fig. 3) indicating that β1 integrins are dispensable for LASVGP-mediated cell attachment and entry.
Fig. 3.
Infection of LASV pseudotypes is independent on β1 integrins. DG (−/−) and DG (+/−), β1 integrin (−/−) and β1 integrin (+/−) mouse ES cells cultured in 96-well plates were infected with the retroviral pseudotypes bearing the GPs of LASV or AMPV (moi = 10). Infection was assessed after 48 h by luciferase assay (n = 3, ±SD).
Attachment of inactivated LASV and LASV pseudotypes to cells perturbs activation of MEK/ERK signalling by laminin
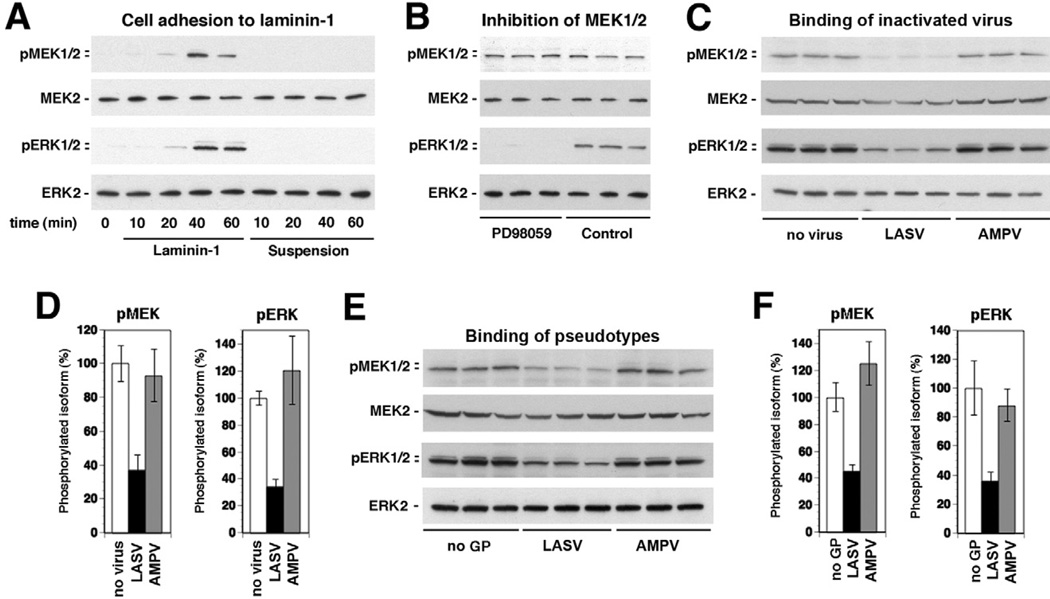
Previous studies demonstrated that cell adhesion to laminin results in activation of the MEK/ERK pathway via β1 integrins, which is counterbalanced by DG (Ferletta et al., 2003). To validate our experimental system, we monitored the activation of the MEK/ERK pathway in WI-26 VA4 cells in response to cell adhesion to laminin employing a cell adhesion assay described previously (Ferletta et al., 2003). Briefly, WI-26 VA4 cells were cultured for 16 h under serum starvation, detached and single-cell suspension prepared. Cells were then added to culture dishes coated with purified mouse laminin-1 or left in suspension. At different time points, cells were lysed, total protein extracted and the phosphorylation of MEK and ERK detected by specific antibodies to the phosphorylated forms of the kinases in Western blot. For normalization, total MEK/ERK was detected with antibodies that are insensitive to the phosphorylation state. Adhesion of WI-26 VA4 cells to laminin-1 resulted in the induction of phosphorylation of MEK and ERK with maximal activation after 40–60 min, a kinetic profile consistent with published work (Ferletta et al., 2003) (Fig. 4A). Addition of the specific MEK inhibitor PD98059 abrogated phosphorylation of ERK, confirming that the enhanced phosphorylation of ERK was mediated by MEK (Fig. 4B).
Fig. 4. Binding of inactivated LASV to cells perturbs laminin-induced activation of the ERK-MAP kinase pathway.
A. Phosphorylation of MEK and ERK in WI-26 VA4 cells in response to cell adhesion to laminin-1. Serum-starved cells were detached and seeded onto wells coated with laminin-1 or kept in suspension. At the indicated time points, total-cell lysates were prepared and subsequently analysed for the presence of MEK1/2 and ERK1/2 as well as the phosphorylated forms of the kinases using specific antibodies.
B. ERK is phosphorylated via MEK: suspensions of serum-starved cells were incubated with the MEK-specific inhibitor PD98059 (50 µM) or solvent control for 30 min and then plated onto wells coated with laminin-1 for 40 min. The phosphorylated forms of MEK1/2 and ERK1/2, MEK2 and ERK2 were detected in total-cell lysates.
C. Binding of inactivated LASV to cells reduces laminin-induced activation of MEK and ERK: serum-starved WI-26 VA4 cells were detached, mixed with inactivated LASV or AMPV (10 particles per cell), or no virus, and plated onto wells coated with laminin-1. After 40 min of cell adhesion, total-cell lysates were prepared and analysed for the presence of phosphorylated MEK and ERK as in (A).
D. Quantification of (C): the signal for phosphorylated MEK1/2 and ERK1/2 in the control samples set as 100% (n = 3, ±SD).
E and F. Binding of LASV pseudotypes reduces laminin-induced activation of MEK and ERK: experiment was performed as in (C) and (D) using retroviral pseudotypes of LASV and AMPV (10 PFU per cell) and pseudotypes without GP (no GP).
Next, we addressed the impact of LASV binding to cellular α-DG on laminin-induced signalling. For this purpose, single-cell suspensions of WI-26 VA4 cells were mixed with inactivated LASV or AMPV (10 particles per cell) immediately before adding to plates coated with laminin-1. The presence of the viruses did not affect the number of adherent cells (data not shown), excluding simple blocking of cell adhesion by the virus. After 40 min, cells were lysed and the phosphorylation of MEK and ERK detected. As shown in Fig. 4C, cell adhesion to laminin-1 in presence of LASV, but not AMPV resulted in significantly reduced activation of MEK and ERK phosphorylation (Fig. 4C and D).
The MEK/ERK kinase pathway is influenced by many cellular signalling cascades including cellular responses to stress. To exclude artefacts due to unknown contaminations that may be present in our inactivated virus preparations, we performed analogous experiments with retroviral pseudotypes for LASV and AMPV. As shown in Fig. 4E and F, adhesion of WI-26 VA4 cells to laminin in presence of pseudotypes gave similar results than obtained with inactivated viruses. Only the presence of pseudotypes bearing the GP of LASV, but not AMPV significantly reduced the induction of MEK/ERK phosphorylation in response to cell adhesion to laminin. Together, the data suggest that LASV attachment to cells somehow perturbs laminin-induced activation of the MEK/ERK pathway.
Activity of the MEK/ERK pathway is dispensable for cell entry of LASV pseudotypes
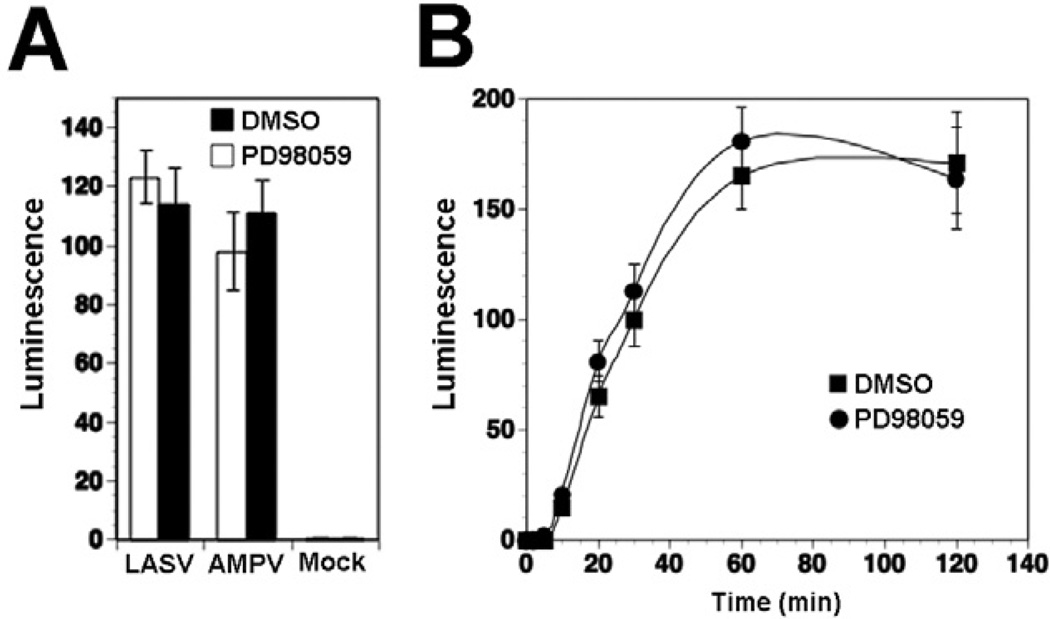
The ability of LASV to modulate cellular MEK/ERK signalling raised the possibility that this pathway may be involved in cell entry of the virus. To test this possibility, we pre-treated cells with the MEK inhibitor PD98059 at concentrations that abrogated ERK phosphorylation (Fig. 4B), followed by infection with LASV and AMPV pseudotypes. When assessed after 48 h, pre-treatment with the MEK inhibitor did not significantly affect LASV pseudotype infection, indicating that MEK/ERK signalling is dispensable for LASVGP-mediated cell attachment and entry (Fig. 5A). Since these first experiments detected infection at 48 h post infection, i.e. at a late time point, we next examined the cell entry kinetics of LASV in presence of the MEK inhibitor PD98059. Upon receptor binding, LASV is taken up by clathrin- and caveolin-independent endocytosis and rapidly delivered to late endosomes, where low pH-dependent membrane fusion occurs (Borrow and Oldstone, 1994; Quirin et al., 2008; Rojek et al., 2008a,b). To assess how fast receptor-bound LASV pseudotypes trafficked to late endosomes, we determined the time required for the virus to become resistant to the lysosomotropic agent ammonium chloride. When added to cells, ammonium chloride raises the endosomal pH rapidly and blocks low pH-dependent membrane fusion without causing overall cytotoxicity (Ohkuma and Poole, 1978; 1981). WI-26 VA4 cells were either pre-treated with PD980592 for 1 h or mock treated with vehicle (DMSO) only. Cells were then incubated with LASV pseudotypes in the cold, to allow virus attachment without internalization. Unbound virus was removed and cells quickly shifted to 37°C to allow virus internalization in presence or absence of PD980592. After different time points, 20 mM ammonium chloride was added to cells and kept throughout the experiment. After 24 h, cells were fixed and infection assessed by detection of the luciferase reporter activity. As shown in Fig. 5B, pre-treatment of cells with PD98059 had no significant effects on the cell entry kinetics of LASV pseudotypes, suggesting that activity of the MEK/ ERK pathway is dispensable for virus cell entry.
Fig. 5. MEK1 activity is dispensable for cell entry of LASV pseudotypes.
A. Infection of LASV pseudotypes is not affected by the MEK inhibitor PD98059. Monolayers of WI-26 VA4 cells were pre-treated with PD98059 (50 µM) or solvent control (DMSO) for 30 min, followed by infection with LASV and AMPV pseudotypes (moi = 0.1). Infection was detected by luciferase assay and fold increase of luminescence above background is given (means ± SD, n = 3).
B. The MEK inhibitor PD98059 does not affect LASV cell entry kinetics. Monolayers of WI-26 VA4 cells were pre-treated with PD98059 as in (A), followed by incubation with LASV pseudotypes (moi = 0.1) in the cold in presence of the drug. After 1 h, unbound virus was removed and pre-warmed (37°C) medium containing the drug added. At the indicated time points, 20 mM ammonium chloride was added and left throughout the experiment. At 24 h post infection was detected by luciferase assay as in (A) (means ± SD, n = 3). Please note that the apparent differences in infection at 60 min were not statistically significant.
Binding of LASV pseudotypes to cells perturbs activation of MEK and ERK via the integrin α6β1
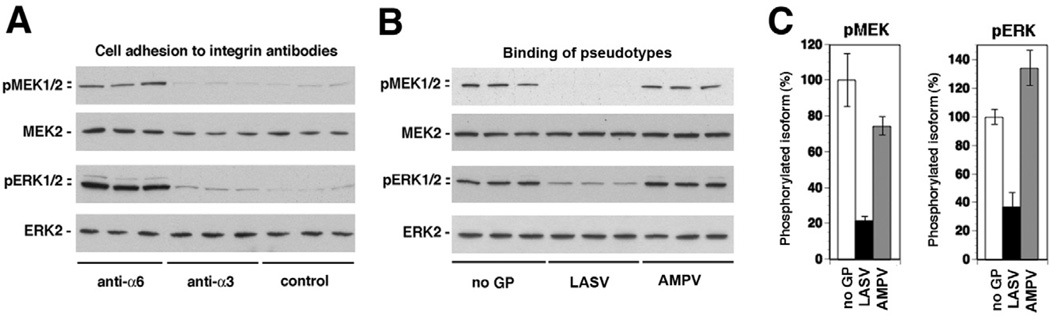
The major laminin-binding integrins in WI-26 VA4 cells are the integrins α6β1 and α3β1, whereas α6β4 integrin is present only at low levels (Ferletta et al., 2003). Since mouse laminin-1 binds only to human α6β1 but not α3β1 integrins (Delwel et al., 1994), laminin-1-induced activation of MEK and ERK in WI-26 VA4 cells is thought to be mediated by α6β1 integrins (Ferletta et al., 2003). To validate the role of α6β1 integrins in laminin-1-induced MEK/ERK signalling in our system, serum-starved WI-26 VA4 cells were added to tissue culture plates coated with the signalling inducing anti-integrin α6 mAb GoH3, as well as mAb P1B5 anti-α3. Consistent with published reports, only adhesion to the mAb anti-integrin α6, but not anti-integrin α3 resulted in significant activation of MEK and ERK (Fig. 6A), confirming the role of α6β1 integrins in laminin-mediated MEK/ERK activation.
Fig. 6. Binding of LASV pseudotypes to cells blocks α6β1 integrin-mediated activation of MEK and ERK.
A. Induction of MEK and ERK phosphorylation by anti-α6 integrin antibody. Serum-starved WI-26 VA4 cells were detached and plated onto wells coated with antibodies to α6 and α3 integrin or an isotype control antibody for 40 min. Activation of MEK and ERK was determined by Western blot.
B. Binding of LASV pseudotypes reduces MEK and ERK phosphorylation induced by anti-α6 integrin antibody: serum-starved WI-26 VA4 cell suspensions were mixed with pseudotypes of LASV and AMPV at 10 particles per cell or control pseudotypes containing no GP (no GP) and plated onto wells coated with antibodies to α6. After 40 min of cell adhesion total-cell lysate were prepared and phosphorylation of MEK and ERK analysed by Western blot.
C. Quantification of (B), setting the signal for phosphorylated MEK1/2 and ERK1/2 in the control sample (no GP) as 100% (n = 3, ±SD).
To specifically address the effect of LASV binding to cells on the activation of MEK/ERK via α6β1 integrin-mediated signalling, we performed cell adhesion to mAb anti-α6 in presence of LASV and MAPV pseudotypes. Single-cell suspensions of serum-starved WI-26 VA4 cells were mixed with LASV and MAPV pseudotypes at 10 particles per cell and immediately added to cell culture plates coated with mAb GoH3 anti-α6 integrin At 40 min, cells were lysed and phosphorylation of MEK and ERK detected by Western blot. Pseudotypes of LASV, but not AMPV specifically perturbed the activation of MEK and ERK induced by mAb anti-α6 (Fig. 6B and C), suggesting that LASVGP binding to cells perturbs activation of MEK and ERK via the integrin α6β1.
LASV pseudotype-induced perturbation of α6β1 integrin-mediated signalling depends on functional DG
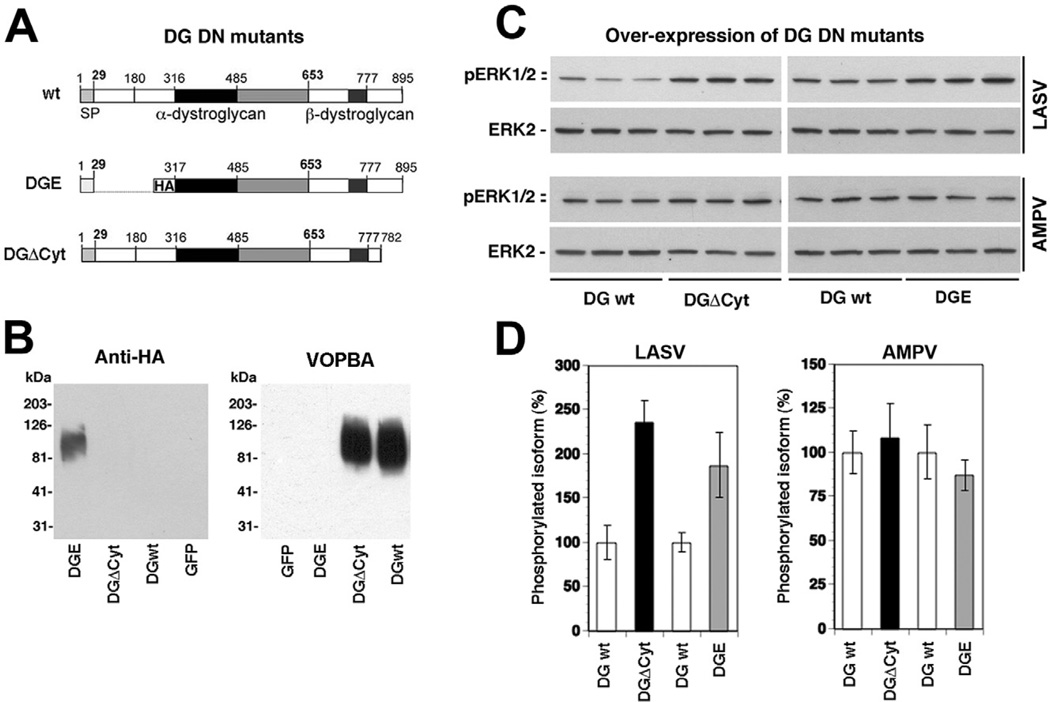
To address the involvement of DG in the LASV-induced perturbation of α6β1 integrin signalling, we utilized two well-characterized DG mutants (Fig. 7A). DGE contains the LASV binding site but lacks the N-terminal domain and does not undergo the post-translational modifications required for LASV binding (Kanagawa et al., 2004). DGΔCyt lacks the cytoplasmic tail of β-DG, but shows normal cell surface expression and virus binding (Kunz et al., 2003). First, we verified virus-binding phenotypes of DGE and DGΔCyt. For this purpose, the DG mutants and wild-type DG were expressed in DG (−/−) ES cells using adenoviral (AdV) vectors (Kunz et al., 2001). After 48 h, total-cell lysates were prepared and glycoproteins isolated by wheat germ agglutinin (WGA) affinity purification (Michele et al., 2002). Glycoprotein fractions were separated by SDS-PAGE and virus overlay assay performed with inactivated LASV (Cao et al., 1998). As expected, the α-DG part of DGE lacked detectable virus binding, whereas α-DG from DGΔCyt and wild-type DG bound LASV with high affinity (Fig. 7B).
Fig. 7. Functional α-DG and the cytoplasmic domain of β-DG are involved in LASV pseudotype-induced inhibition of α6 integrin-mediated MEK/ERK activation.
A. Schematic representation of the DG mutants.
B. Detection of the α-DG parts of the DG variants. DGE, DGΔCyt, wild-type DG and GFP were expressed in DG (−/−) ES cells using AdV vectors. After 48 h, total membrane extracts were prepared and probed with an antibody to HA and in virus overlay protein binding assay (VOPBA).
C. Overexpression of DG DN mutants releases LASV pseudotype-induced inhibition of α6 integrin mediated MEK/ERK activation. WI-26 VA4 cells were infected with AdV expressing the DG variants. After 48 h, cells were serum-starved and detached. Single-cell suspensions were mixed with LASV and AMPV pseudotypes and plated onto wells coated with antibody to α6. Activation of ERK was determined by Western blot.
D. Quantification of (B), setting the signal for phosphorylated MEK1/2 and ERK1/2 in the control sample (no GP) as 100% (n = 3, ±SD).
To test the effect of the DG mutants on LASV-induced modulation of α6β1 integrin signalling, WI-26 VA4 cells were infected with AdV vectors expressing DGE, DGΔCyt and wild-type DG. After 48 h, cells were serum-starved for 16 h, detached, and added to plates pre-coated with mAb anti-a6 integrin in presence of LASV and AMPV pseudotypes. Cells were lysed and phosphorylation of ERK assessed by Western blot. In cells overexpressing DGE and DGΔCyt the inhibition of anti-α6 integrin-induced ERK phosphorylation by LASV pseudotypes was significantly released (Fig. 7C and D). As expected, no effect was observed in cells exposed to AMPV pseudotypes (Fig. 7C and D). The ability of the DG mutants to partially release the LASV-induced blocking of α6β1 integrin-mediated MEK/ERK activation suggest that LASV-pseudotype induced perturbation of α6β1 integrin-mediated signalling involves functional DG.
Discussion
Viruses have evolved to use a plethora of different cell surface molecules with very different biochemical characteristics and functions for host cell attachment and entry (Smith and Helenius, 2004; Marsh and Helenius, 2006). In some cases, the pathogens evolved to mimic the nature of the natural ligands of their cellular receptors, like, e.g. the integrin binding sites found in some picornaviruses (Baranowski et al., 2003). Old World arenaviruses, including LASV provide a striking example for this phenomenon (Rojek et al., 2007a; Oldstone and Campbell, 2011). Similar to DG’s ECM ligands, high-affinity binding of arenaviruses critically depends on functional glycans present on α-DG. A comparative study between ECM proteins and arenaviruses revealed that the viruses mimic the molecular mechanism of receptor binding of α-DG’s ECM ligands in a striking manner as both recognizing a highly conserved glycan epitope derived from the glycosyltransferase LARGE (Rojek et al., 2007a). In the present study we investigated the consequences of this ‘mimicry’ on the normal function of DG in the host cell, in particular DG-mediated signal transduction.
Several lines of evidence indicate that in the host cell, DG can associate with signalling molecules, including components of the MEK/ERK signalling pathway, Grb2, MEK and ERK (Yang et al., 1995; Spence et al., 2004; Moore and Winder, 2010). However the roles of these interactions for the cellular function of DG are currently unclear. Engagement of cellular α-DG by LASVGP, displayed on either inactivated LASV virions or retroviral pseudotypes, resulted in significant recruitment of Grb2 and MEK1 by the DG complex. Interestingly, we were unable to detect recruitment of the GTP exchange factor Sos, required by Grb2 for the activation of the canonical Ras/Raf/MEK/ERK pathway and found no evidence for activation of MEK/ERK signalling upon virus binding at any point, suggesting recruitment of Grb2 and MEK1 into an inactive signalling complex. An important role for such an inactive signalling complex for viral entry seemed rather unlikely. We therefore investigated the effect of these virus-induced changes on DG’s association with Grb2 and MEK1 on the known cross-talk between DG and β1 integrins.
Previous work by us and others showed that attachment and cell entry of LASV critically depends on the high-affinity interaction between LASVGP and cellular α-DG (Cao et al., 1998; Kunz et al., 2005a; Reignier et al., 2006). Our present study revealed that β1 integrins are dispensable for attachment and cell entry of LASV pseudotypes. However, binding of inactivated LASV and LASV pseudotypes to cells markedly reduced the phosphorylation of MEK and ERK in response to laminin-1 and signalling activating antibodies to α6β1 integrins. Using mutants of DG, which either were deficient in virus binding or lacked the cytoplasmic tail of β-DG, we found that the effect of LASV cell attachment on α6β1 integrin signalling involves functional DG. Together, our data provide evidence that high-affinity LASV binding to cellular α-DG perturbs the signalling cross-talk between DG and α6β1 integrins, shifting the normal signalling equilibrium towards inhibition of the MEK/ERK pathway.
It is currently unclear how LASV binding to α-DG modulates MEK/ERK signalling through α6β1 integrins. To activate ERK, integrins can use two distinct pathways, one, involving FAK, and the other, the adaptor protein Shc (Giancotti and Ruoslahti, 1999). In both pathways, tyrosine phosphorylation of the adaptor proteins generates binding sites for the SH2 domain of Grb2, which in turn recruits Sos leading to activation of the Ras/Raf/MEK/ ERK cascade. In contrast, binding of Grb2 to β-DG occurs in an SH3-dependent manner (Yang et al., 1995). Here we show that virus binding affects the association of DG with Grb2. However, an association of DG with Grb2 and MEK1 is already detected in uninfected cells and the extent of recruitment upon virus binding seems modest (Fig. 2F). It seems therefore rather unlikely that virus–receptor binding can sufficiently sequester signalling molecules like Grb2 and MEK1 to affect α6β1 integrin signalling. One might speculate that LASV binding to DG induces a negative regulatory signal of unknown nature that may affect α6β1 integrin-induced MEK/ERK signalling (Fig. 8).
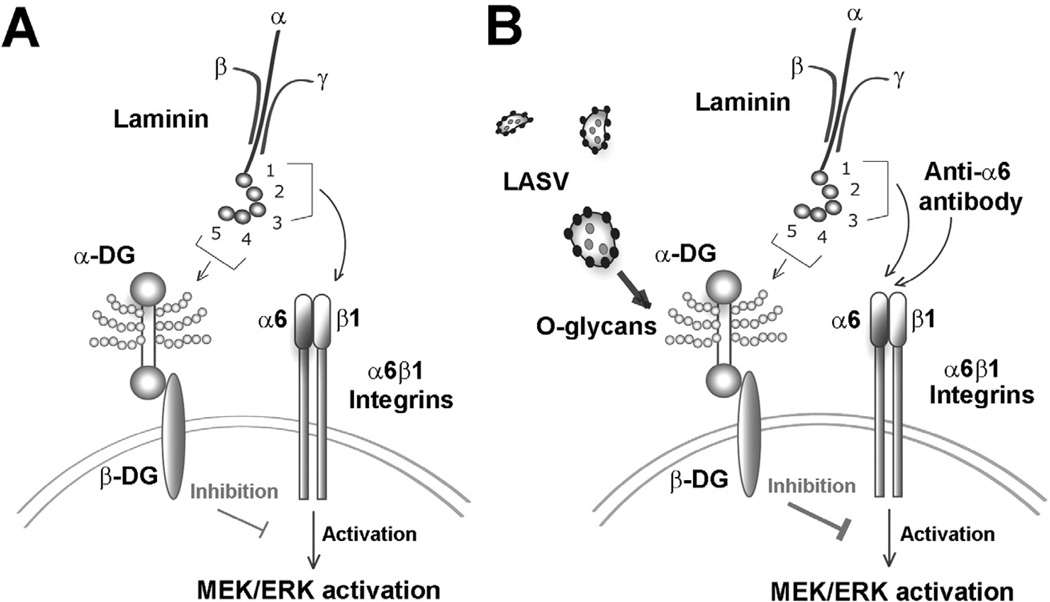
Fig. 8. Working model: binding of LASV to DG perturbs signalling cross-talk with β1 integrins.
A. Binding of laminin to cellular DG modulates MEK/ERK signalling through α6β1 integrins: laminin engages cellular DG via the LG domains 4 and 5 of the α1 chain (Hohenester et al., 1999) and binding critically depends on α-DG-linked O-glycans, in particular sugar polymers attached by LARGE (Kanagawa et al., 2004). Binding of laminin to α6β1 integrins involves the LG domains 1–3 of α1 (Hohenester et al., 1999) and results in activation of MEK/ERK signalling (Ferletta et al., 2003). Simultaneous binding of laminin to DG via LG4/5 inhibits activation of MEK/ERK via α6β1 integrins (Ferletta et al., 2003).
B. High-affinity binding of LASV to cellular α-DG perturbs the signalling cross-talk between DG and α6β1 integrins (this study): binding of inactivated LASV and LASV pseudotypes to cellular DG inhibits activation of α6β1 integrins via laminin or a signalling-inducing antibody to α6. The effect of LASV on α6β1 integrin signalling depends on the functional O-glycosylation of α-DG and the cytoplasmic domain of β-DG. The nature of the inhibitory signal induced by binding of laminin LG4/5 and LASV is currently unknown.
Intrigued by the observation that binding of LASV to cells can modulate cellular MEK/ERK signalling, we investigated the role of this pathway for LASV cell entry. Inhibition of MEK, which is the only kinase that can phosphorylate ERK, did not affect infection of cells with LASV pseudotypes, making a role of this pathway in LASVGP-mediated cell entry rather unlikely. Due to biosafety restrictions associated with work with live LASV, a possible role of MEK/ERK signalling in post-entry steps of LASV replication have not yet been addressed, but will be pursued in future studies.
Since DG and α6β1 integrins are coexpressed on a wide variety of human cell types involved in LASV pathogenesis like epithelial cells, endothelial cells and macrophages (Wei et al., 1998; ffrench-Constant and Colognato, 2004) the impact of LASV binding on DG-mediated signalling may affect normal cell function in LASV infected individuals. A possible concern with our in vitro studies is the relatively high particle/cell ratios used. However, in late stages of fatal human Lassa fever, virus loads often exceed 109 infectious particles per ml of blood and similar virus loads are found in many tissues (Walker et al., 1982; McCormick and Fisher-Hoch, 2002; Moraz and Kunz, 2011). In this situation, extensive binding of virus particles to cellular α-DG may result in significant perturbation of DG-mediated signalling that may contribute to cellular dysfunctions that are associated with the Lassa shock syndrome.
In fatal Lassa fever there is surprisingly little inflammation and tissue destruction, vascular damage is mild and disseminated intravascular coagulation (DIC) is rare (Walker et al., 1982; McCormick and Fisher-Hoch, 2002). The absence of classical hallmarks of immunopathology in fatal disease suggests that the direct interaction of the virus with host cells may contribute to some aspects of pathogenesis, such as vascular leakage and oedema formation. Among other mechanisms, the virus-induced perturbation of ECM-induced cell signalling shown in this study, may contribute to the functional alterations of epithelial and vascular endothelial cells that precede shock and death (Fisher-Hoch et al., 1987). This type of LASV-induced receptor signalling reported here is likely due to the extensive mimicry of endogenous ligand binding by the pathogen. The consequent perturbation of cell signalling appears as a ‘collateral damage’ inflicted on the cell that may contribute to viral pathogenesis.
Experimental procedures
Proteins and antibodies
Mouse laminin-1 was from Gibco-BRL (Gaithersburg, MD). Monoclonal antibody (mAb) IIH6 anti-α-DG has been described (Ervasti and Campbell, 1991). Polyclonal rabbit anti-laminin-1 was from Sigma (St. Louis, MO) and rabbit anti-influenza HA (Y11) and mouse anti-HA (F7) from St. Cruz Biotechnology (St. Cruz, CA). HRP-conjugated secondary Abs and Streptavidin-HRP were from Pierce. MAbs to human integrins P1B5 anti-α3 and GoH3 anti-α6 were from Chemicon (Temecula, CA) and BD Biosciences (San Jose, CA) respectively. Mouse mAbs specific for MEK1, MEK2, ERK1 and ERK2 were from Transduction Laboratories (Lexington, KY), rabbit polyclonal Abs against phospho-MEK1/2 (S217/221) and phospho-ERK1/2 (T202/Y204) were from New England Biolabs (Beverly, MA). Polyclonal rabbit anti-Sos-1 Abs were from Upstate (Lake Placid, NY), mouse mAb anti-Grb2 from Chemicon, and rabbit polyclonal Ab to p125FAK from St. Cruz. The Steady Glo® and Bright-Glo® luciferase assay systems were obtained from Promega (Madison, WI). The MEK-specific inhibitor PD98059 was obtained from Calbiochem.
Cell lines
WI-26 VA4 cells (ATCC CCL-95.1) were cultured in DMEM, 10% (v/v) FBS, supplemented with glutamine, and penicillin/ streptomycin. Embryonic stem (ES) cells DG (+/−), DG (−/−) have been described (Henry and Campbell, 1998). Mouse ES cells β1 integrin (+/−) and β1 integrin (−/−) (Fassler and Meyer, 1995; Fassler et al., 1995) were kindly provided by Dr R. Fassler. All ES cell lines were cultured as described (Henry and Campbell, 1998).
Virus strains, purification and quantification
Recombinant AdV DGΔH30-A316 (DGE) and DGΔCyt and wt DG have been described (Kunz et al., 2001; 2003). Lassa virus (LASV) strain Josiah and Amapari (AMPV) virus were obtained from the collection at the Special Pathogens Branch, Center for Disease Control and Prevention in Atlanta GA and inactivated viruses produced as reported (Spiropoulou et al., 2002). Retroviral pseudotypes expressing GFP and luciferase reporters were produced as described (Rojek et al., 2006) and, where indicated, inactivated by UV irradiation for 5 min. Inactivation was verified by luciferase assay.
Immunoblotting and VOPBA
Proteins were separated by gel electrophoresis and transferred to nitrocellulose. After blocking in 5% (w/v) skim milk in PBS, membranes were incubated with Abs used at following concentrations: mAb IIH6, mAb F7, mAb 8D5 and polyclonal Abs FTP, AP83 and Y11 (10 µg ml−1) in 2% (w/v) skim milk, PBS for 12 h at 6°C. Abs to MEK1/2, ERK1/2, Sos-1, Grb-2, p125FAK, phospho-MEK1/2 and phospho-ERK1 at 1:1000 in 2% (w/v) skim milk, TBS for 12 h at 6°C. Secondary Abs coupled to HRP were applied 1:5000 in PBS, 0.1% (w/v) Tween for 1 h at room temperature. Blots were developed by enhanced chemiluminescence (ECL) using Super Signal West Pico ECL Substrate (Pierce). Laminin overlay assay (LOA) and VOPBA with γ-inactivated LASV, and AMPV were performed as described (Kunz et al., 2005b).
Virus cell binding assay
Monolayers of WI-26 VA4 cells were cultured in 96-well microtitre plates. For virus binding, medium was removed, cells washed twice with PBS, chilled on ice and blocked for 1 h in 1% (w/v) FBS/PBS containing 0.1% (w/v) of sodium azide. Cells were incubated with the indicated concentrations of inactivated LASV and AMPV in 1% (w/v) FBS/PBS/0.1% (w/v) sodium azide. After 1 h on ice, cells were washed three times with cold PBS and fixed with 4% (w/v) paraformaldehyde supplemented with 0.1% (w/v) glutaraldehyde for 20 min in ice. For detection of bound virus, cells were incubated with mAb 83.6 (20 µg ml−1 purified IgG) overnight in the cold. After three washes in PBS, primary antibody was detected with goat anti-mouse IgG conjugated to HRP for 45 min in the cold. After three wash-steps in PBS, HRP-conjugated secondary antibody was detected in a colour reaction using ABTS [2,2′azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] substrate and OD405 detected in an ELISA reader.
Infection of cells with retroviral pseudotypes
Cells were plated in 96-well plates in a density of 104 cells per well. After 24 h, retroviral pseudotypes were added at the indicated moi and incubated for 1 h at 37°C. The viral particles were removed, cells washed twice with DMEM and fresh medium added. Luciferase activity was determined by Steady-Glo® luciferase assay except in murine ES cells. AdV-mediated gene transfer of wild-type DG and the DG DN mutants DGE (DH30-A316) and DGΔCyt was performed as described (Kunz et al., 2005b). After 48 h, the indicated infectious units (iu) of pseudotypes were added to AdV transfected DG (−/−) ES cells, as well as untreated control cells and incubated for 1 h at 37°C. The viral particles were removed, cells washed twice with DMEM and fresh medium added. Infection was quantified by Bright Glo® luciferase assay. Luminescence was calculated as fold increase over background signals obtained from uninfected cells. Cell entry kinetics of LASV pseudotypes were performed as described (Rojek et al., 2008b). Blocking of infection with mAb IIH6 was done as reported (Kunz et al., 2005b).
Co-immunoprecipitation
For virus binding and co-IP of β-DG with signalling molecules, WI-26 VA4 cells were transfected with DGHA and DGwt with Superfect® (QIAGen). After 48 h, cells were exposed to purified, inactivated LASV, AMPV, or LASV- and AMPV pseudotypes at a particle to cell ratio of 1:100 for the indicated time periods. To detect changes in the association of signalling molecules with β-DG, co-IP was performed as described (Rojek et al., 2007b) using either mAb F7 or Y11 immobilized on Sepharose 4B for IP of DHHA and the abovementioned Abs for detection of signalling molecules in Western blot. IP with mouse mAb F7 were analysed with polyclonal rabbit Abs and IP with pAb Y11 with mouse mAbs.
Detection of MEK/ERK activation by cell adhesion to laminin and anti-α6 integrin Abs
Cell adhesion assays were performed according to Ferletta et al., 2003. MEK/ERK activation was detected from WI-26 VA4 cells that had been seeded to confluence, serum starved for 16–24 h, detached and plated on laminin-1 or wells pre-coated with mAbs to a3 and a6 integrins in presence or absence of purified inactivated LASV, AMPV, or LASV- and AMPV pseudotypes at a particle to cell ratio of 1:10 for the indicated time periods. Phosphorylated and total MEK and ERK were determined by Western blot as described above and the degree of phosphorylation determined as described (Ferletta et al., 2003) using densitometry (Kunz et al., 2004). For overexpression of the DN DG mutants DGE and DGΔCyt and wt DG in WI-26 VA4 cells we used AdV vectors (moi = 100) for gene transfer as described (Kunz et al., 2005b).
Acknowledgements
The authors thank Dr Michael B.A. Oldstone for his generous support and Dr R. Fassler for the β1 integrin null cells. The retroviral construct pLZRs-Luc-gfp was kindly provided by Dr Gary Nabel. This research was supported by the Prix Leenaards 2009 pour la promotion de la recherche scientifique (2009) (S.K. and F.G.V.d.G.), Swiss National Science Foundation Grant FN 310030_132844 (S.K.), the Marie Curie International Reintegration Grant No. 224780 of the European Community (S.K.), and Grant 1U54 AI065359 of the Pacific Southwest Regional Center of Excellence for Biodefense and Emerging Infectious Disease (S.K.). K.P.C. is an investigator of the Howard Hughes Medical Institute.
References
- Baranowski E, Ruiz-Jarabo CM, Pariente N, Verdaguer N, Domingo E. Evolution of cell recognition by viruses: a source of biological novelty with medical implications. Adv Virus Res. 2003;62:19–111. doi: 10.1016/S0065-3527(03)62002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovicio SA, Satz JS, et al. LARGE can functionally bypass alpha-dystrolycan glycosylation defects in distinct congential muscular dystrophy. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- Beyer WR, Popplau D, Garten W, von Laer D, Lenz O. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J Virol. 2003;77:2866–2872. doi: 10.1128/JVI.77.5.2866-2872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Oldstone MB. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- Buchmeier MJ, Torre JC, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DL, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven; 2007. pp. 1791–1828. [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, et al. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus [see comments] Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Cavaldesi M, Macchia G, Barca S, Defilippi P, Tarone G, Petrucci TC. Association of the dystroglycan complex isolated from bovine brain synaptosomes with proteins involved in signal transduction. J Neurochem. 1999;72:1648–1655. doi: 10.1046/j.1471-4159.1999.721648.x. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, et al. Distinct and overlapping ligand specificities of the alpha 3Abeta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschli B, Quirin K, Wepf A, Weber J, Zinkernagel R, Hengartner H. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J Virol. 2006;80:5897–5907. doi: 10.1128/JVI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fassler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferletta M, Kikkawa Y, Yu H, Talts JF, Durbeej M, Sonnenberg A, et al. Opposing roles of integrin alpha6Abeta1 and dystroglycan in laminin-mediated extracellular signal-regulated kinase activation. Mol Biol Cell. 2003;14:2088–2103. doi: 10.1091/mbc.E03-01-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Hoch SP, Mitchell SW, Sasso DR, Lange JV, Ramsey R, McCormick JB. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J Infect Dis. 1987;155:465–474. doi: 10.1093/infdis/155.3.465. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- Henry MD, Satz JS, Brakebusch C, Costell M, Gustafs-son E, Fassler R, Campbell KP. Distinct roles for dystroglycan, beta1 integrin and perlecan in cell surface laminin organization. J Cell Sci. 2001;114:1137–1144. doi: 10.1242/jcs.114.6.1137. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Tisi D, Talts JF, Timpl R. The crystal structure of a laminin G-like module reveals the molecular basis of alpha-dystroglycan binding to laminins, perlecan, and agrin. Mol Cell. 1999;4:783–792. doi: 10.1016/s1097-2765(00)80388-3. [DOI] [PubMed] [Google Scholar]
- Igonet S, Vaney MC, Vonhrein C, Bricogne G, Stura EA, Hengartner H, et al. X-ray structure of the arenavirus glycoprotein GP2 in its postfusion hairpin conformation. Proc Natl Acad Sci USA. 2011;108:19967–19972. doi: 10.1073/pnas.1108910108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell–matrix linkage in the pathogenesis. J Hum Genet. 2006;13:13. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, et al. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kunz S. The role of the vascular endothelium in arenavirus haemorrhagic fevers. Thromb Haemost. 2009;102:1024–1029. doi: 10.1160/TH09-06-0357. [DOI] [PubMed] [Google Scholar]
- Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J Cell Biol. 2001;155:301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Campbell KP, Oldstone MB. Alpha-dystroglycan can mediate arenavirus infection in the absence of beta-dystroglycan. Virology. 2003;316:213–220. doi: 10.1016/j.virol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Kunz S, Calder L, Oldstone MB. Electron microscopy of an alpha-dystroglycan fragment containing receptor sites for lymphocytic choriomeningitis virus and laminin, and use of the receptoid body as a reagent to neutralize virus. Virology. 2004;325:207–215. doi: 10.1016/j.virol.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Kunz S, Rojek J, Perez M, Spiropoulou C, Oldstone MB. Characterization of the interaction of Lassa fever virus with its cellular receptor α-dystroglycan. J Virol. 2005a;79:5979–5987. doi: 10.1128/JVI.79.10.5979-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Rojek J, Spiropoulou C, Barresi R, Campbell KP, Oldstone MB. Post-translational modification of alpha-dystroglycan, the cellular receptor for arenaviruses by the glycosyltransferase LARGE is critical for virus binding. J Virol. 2005b;79:14282–14296. doi: 10.1128/JVI.79.22.14282-14296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz O, ter Meulen J, Klenk HD, Seidah NG, Garten W. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc Natl Acad Sci USA. 2001;98:12701–12705. doi: 10.1073/pnas.221447598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, et al. Post-translational disruption of dystroglycan–ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Winder SJ. Dystroglycan versatility in cell adhesion: a tale of multiple motifs. Cell Commun Signal. 2010;8:3. doi: 10.1186/1478-811X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraz ML, Kunz S. Pathogenesis of arenavirus hemorrhagic fevers. Expert Rev Anti Infect Ther. 2011;9:49–59. doi: 10.1586/eri.10.142. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysos-omes of weakly basic substances. J Cell Biol. 1981;90:656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB, Campbell KP. Decoding arenavirus pathogenesis: essential roles for alpha-dystroglycan–virus interactions and the immune response. Virology. 2011;411:170–179. doi: 10.1016/j.virol.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin K, Eschli B, Scheu I, Poort L, Kartenbeck J, Helenius A. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology. 2008;378:21–33. doi: 10.1016/j.virol.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Reignier T, Oldenburg J, Noble B, Lamb E, Romanowski V, Buchmeier MJ, Cannon PM. Receptor use by pathogenic arenaviruses. Virology. 2006;353:111–120. doi: 10.1016/j.virol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Rojek JM, Spiropoulou CF, Kunz S. Characterization of the cellular receptors for the South American hemorrhagic fever viruses Junin, Guanarito, and Machupo. Virology. 2006;349:476–491. doi: 10.1016/j.virol.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Rojek JM, Spiropoulou CF, Campbell KP, Kunz S. Old World and Clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by {alpha}-dystroglycan’s host-derived ligands. J Virol. 2007a;81:5685–5695. doi: 10.1128/JVI.02574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Campbell KP, Oldstone MB, Kunz S. Old World arenavirus infection interferes with the expression of functional {alpha}-dystroglycan in the host cell. Mol Biol Cell. 2007b;29:29. doi: 10.1091/mbc.E07-04-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Perez M, Kunz S. Cellular entry of lymphocytic choriomeningitis virus. J Virol. 2008a;82:1505–1517. doi: 10.1128/JVI.01331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Sanchez AB, Nguyen NT, de la Torre JC, Kunz S. Different mechanisms of cell entry by human-pathogenic Old World and New World arenavi-ruses. J Virol. 2008b;82:7677–7687. doi: 10.1128/JVI.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Lee AM, Nguyen N, Spiropoulou CF, Kunz S. Site 1 protease is required for proteolytic processing of the glycoproteins of the South American hemorrhagic fever viruses Junin, Machupo, and Guanarito. J Virol. 2008c;82:6045–6051. doi: 10.1128/JVI.02392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M, Stroher U, Ebihara H, Feldmann H, Kawaoka Y. Identification of cell surface molecules involved in dystroglycan-independent Lassa virus cell entry. J Virol. 2012;86:2067–2078. doi: 10.1128/JVI.06451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- Spence HJ, Dhillon AS, James M, Winder SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5:484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, McCormick JB, Johnson KM, Webb PA, Komba-Kono G, Elliott LH, Gardner JJ. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol. 1982;107:349–356. [PMC free article] [PubMed] [Google Scholar]
- Weber EL, Buchmeier MJ. Fine mapping of a peptide sequence containing an antigenic site conserved among arenaviruses. Virology. 1988;164:30–38. doi: 10.1016/0042-6822(88)90616-2. [DOI] [PubMed] [Google Scholar]
- Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the alpha6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP. SH3 domain-mediated interaction of dystroglycan and Grb2. J Biol Chem. 1995;270:11711–11714. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]