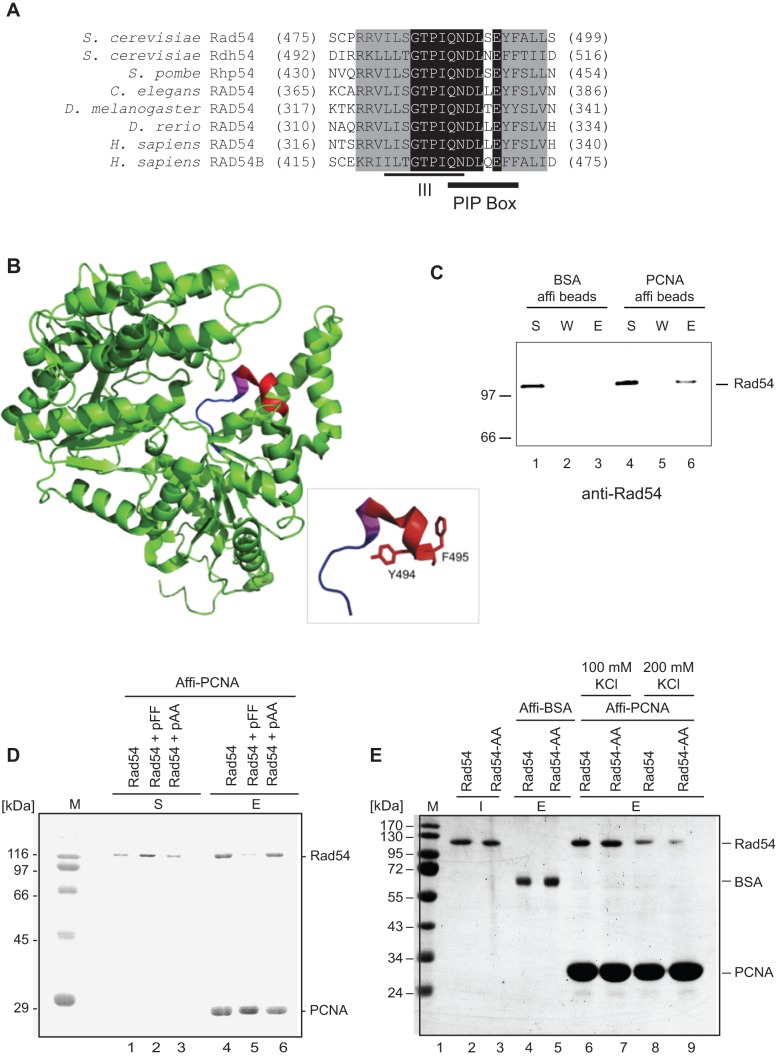
Figure 1. The Rad54 family contains a conserved PCNA interaction motif, and yeast Rad54 directly interacts with PCNA.
A. Alignment of the Rad54 family of proteins. The conservation of the amino acid sequence around motif III in Rad54 family members from budding yeast to human is depicted. Amino acids shaded in black are identical between the sequences, while grayed amino acids retain highly similar side chains. The location of the PCNA interaction protein box (PIP-box) is shown below the alignment. The two C-terminal highly-conserved aromatic residues were changed to alanines by site-directed mutagenesis to create the Rad54-AA (Y494A, F495A) mutant protein. B. Section of the Rad54 structure containing the PIP box and ATPase domains. The tertiary structure of the protein Rad54 from D. rerio (Zebrafish (PDB ID 1Z3I)) is represented in the ribbon diagram. Motif III (317-ISGTPIQN-324, corresponding to amino acids 481–489 in S. cerevisiae Rad54) is shown in blue, the PIP-box motif (323-QNDLLEYF-330, corresponding to amino acids 488–495 in S. c. Rad54) is shown in red, while overlapping residues assigned to both motifs (323-QN-324) are shown in magenta. The detailed view (inset) shows these two motifs and mutated residues Y329 (494) and F330 (495) in stick representation. C. Rad54 interacts with PCNA in vitro. Rad54 protein was mixed with either control beads (affi-BSA, lanes 1–3) or with PCNA immobilized on affi-beads (lanes 4–6). After incubation for 30 min at 4°C with occasional mixing, the reaction mixtures were centrifuged and the supernatant was separated from the beads. Next, the beads were washed and the proteins were eluted by SDS-PAGE loading dye. Supernatant (S), wash (W) and eluate (E) fractions were separated on a 12% SDS-PAGE gel, followed by blotting and detection with α-Rad54 antibodies. D. Oligopeptides derived from the PIP-box outcompete PCNA for interaction with Rad54. In the pull-down experiments shown, Rad54 was mixed with immobilized PCNA in the presence of an oligopeptide derived from the Rad54 PIP box (pFF; lanes 2, 5) or its mutated version (pAA; lanes 3, 6). Lanes 1, 4 represent control experiment where no peptide was added. The reactions were performed as described in C. Supernatant (S), and eluate (E) fractions were separated on a 12% SDS-PAGE gel, followed by Coomassie staining. E. PCNA interaction with Rad54 and Rad54-AA. In these pull-down experiments, Rad54 (lanes 6 and 8) or Rad54-AA (lanes 7 and 9) were mixed with PCNA immobilized on affi-beads, or with BSA affi-beads as a control (lanes 4, 5). The reactions were performed as described in C. Input (I) and eluate (E) fractions were separated on a 12% SDS-PAGE gel, followed by Coomassie staining.