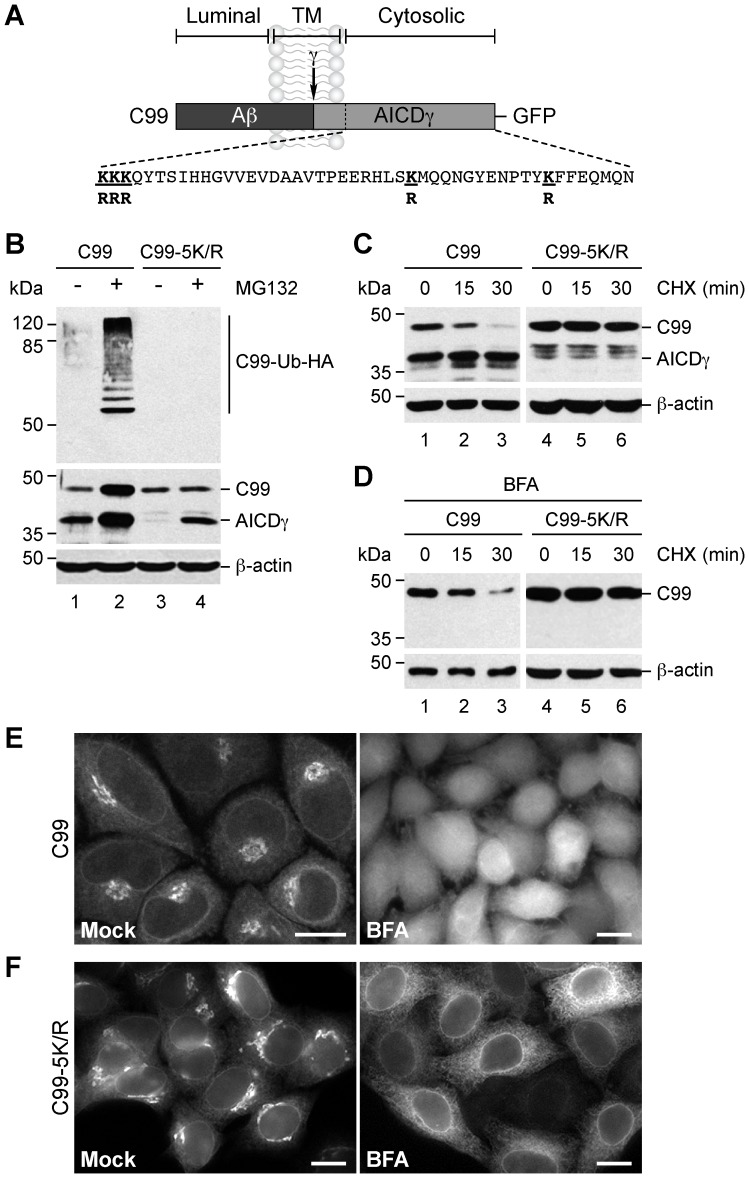
Figure 5. Degradation of C99 after redistribution to the endoplasmic reticulum requires polyubiquitination of its cytosolic lysine residues.
(A) Schematic representation of GFP-tagged C99 indicating its topological domains, the position of the Aβ peptide, the γ-secretase cleavage site, the AICDγ fragment, and the sequence of the cytosolic tail highlighting the substitutions in its five lysine residues (bold underline). (B–D) H4 cells stably expressing GFP-tagged C99-F/P-D/A (C99) or C99-5K/R-F/P-D/A (C99-5K/R) were processed as follows: (B) transfected with HA-tagged ubiquitin and left untreated or treated with 1 µM MG132 for 4 h, and after denaturation soluble extracts immunoprecipitated with anti-GFP antibody; (C) incubated with 150 µg/ml CHX and 40 µg/ml chloramphenicol for 0–30 min; or (D) pretreated with 5 µg/ml BFA for 1 h before further incubation with BFA and the combination of CHX and chloramphenicol for 0–30 min. Proteins were analyzed by immunoblot with HRP-conjugated anti-HA antibody (B; C99-Ub-HA), or anti-GFP antibody (B–D). Immunoblot with anti-β-actin antibody was used as loading control. The positions of molecular mass markers are indicated on the left. (E–F) Confocal fluorescence microscopy of cells stably expressing GFP-tagged C99 (E) or C99-5K/R (F) left untreated (Control) or treated with 5 µg/ml BFA for 1 h. Bars, 10 µm.