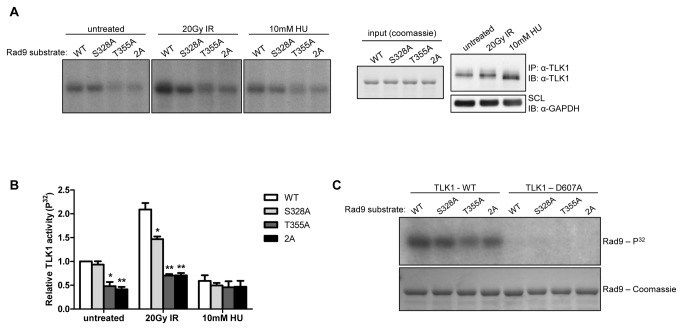
Figure 2. In vitro phosphorylation of full-length GST-Rad9 by TLK1.
TLK1 was immunoprecipitated from HeLa cells and incubated with recombinant full-length GST-Rad9 bearing the indicated point-mutations. A. A representative autoradiograph (left panel) of a dried gel that was subsequently exposed to a phosphor-screen, quantitated, and corrected for background and Rad9 and TLK1 input (middle panel and right panel, respectively. 2A refers to a S328A/T355A double mutant. B. Phosphorylation was quantitated using a Storm 820 phospho-imager. Signal intensity was normalized against the amount of phosphorylation present in the untreated WT reaction. Error bars indicate the standard error of three independent experiments. Asterisks denote statistically significant differences compared to the level of phosphorylation of WT Rad9 within each treatment. p=0.034 (untreated T355A), p=0.004 (untreated 2A), p=0.0252 (IR – S328A), p=0.0067 (IR – T355A), p=0.0053 (IR – 2A). One asterisk denotes p ≤ 0.05. Two asterisks denote p ≤ 0.01. C. Similar to A, full-length GST-Rad9 constructs were incubated with WT and D607A myc-TLK1 immunoprecipitated from HeLa cells.