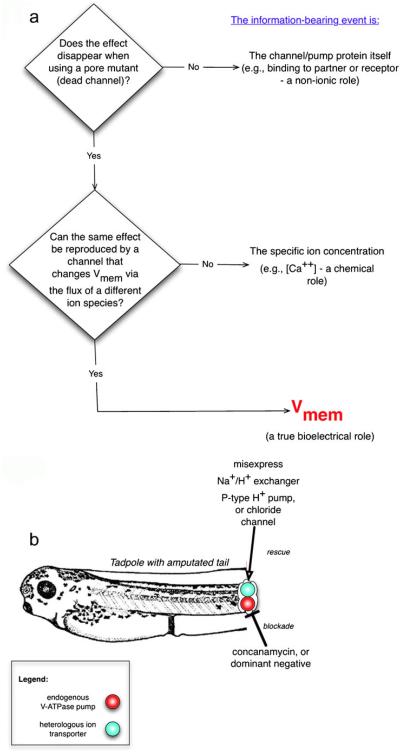
Fig. 6.
Isolation of the information-bearing aspect of an ion-translocator function. In any given assay (process involving cell signaling), the function of a given ion transporter can be dissected by inhibiting the transporter and attempting to rescue the process with several distinct constructs that distinguish among ion-independent roles, ion-specific roles, or voltage-dependent roles (a). If a rescue can be made with an inactive (e.g., pore-mutant) form of the channel, then a non-ion-passing function (e.g., binding partner for some other protein) is implicated. If a rescue can be made with only the same channel family and no others, an ion-specific role is implicated. If any transporter of a different ion but with similar effects on transmembrane potential is sufficient to reproduce the same phenotype, then voltage alone is implicated. An example is shown in b: in the amputated tadpole tail, the native V-ATPase proton pump that is required for regeneration was blocked. Misexpression of a heterologous (yeast) P-type proton pump protein (with no sequence or structure homology to the V-ATPase) rescued regeneration, indicating that the proton pumping, not some ion-independent function, carried the signal necessary to initiate regeneration. In contrast, regeneration was not rescued by the electroneutral sodium/proton exchanger, ruling out the pH gradient as the causal factor in initiating regeneration (instead implicating Vmem regulation as the information-bearing aspect of this process). A Vmem role would be directly demonstrated by misxpressing a chloride channel and controlling intracellular chloride concentration in accordance with the Goldman equation, to reveal the voltage at which regeneration is initiated