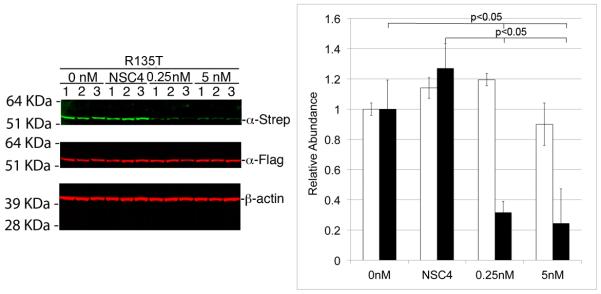
Figure 4. Dual Flag Strep Tag II quantitative infrared immunoblotting confirming allele specific silencing.
AD293 cells were transiently co-transfected with pKRT12-WT/FlagHA (K12-WT) and pKRT12-Arg135Thr/StrepHA (K12-Arg135Thr) and 0, 0.5 or 5 nM K12-Arg135Thr-5 or NSC4 siRNA, immunoblotting of whole cell lysates with α-Flag and α-StrepTagII antibodies and differential fluorescence visualization using a LI-COR Odyssey scanner revealed preferential inhibition of mutant K12 StrepTagII fusion protein synthesis by the K12-Arg135Thr-5 siRNA. Semiquantitative analyses of α-StrepTagII (mutant top panel, green bands) and α-Flag (wild-type, middle panel, red bands) immunoblot signal intensities, relative to β-actin (bottom panel), were used to quantify siRNA inhibition specificities and potencies. Mean relative abundances (n=9) are shown and error bars indicate signal intensity standard deviations. Approximately 70-80% knock down of the mutant allele was noted at both concentrations of siRNA assessed and no knock down was noted with the NSC4 negative control siRNA (p<0.05).