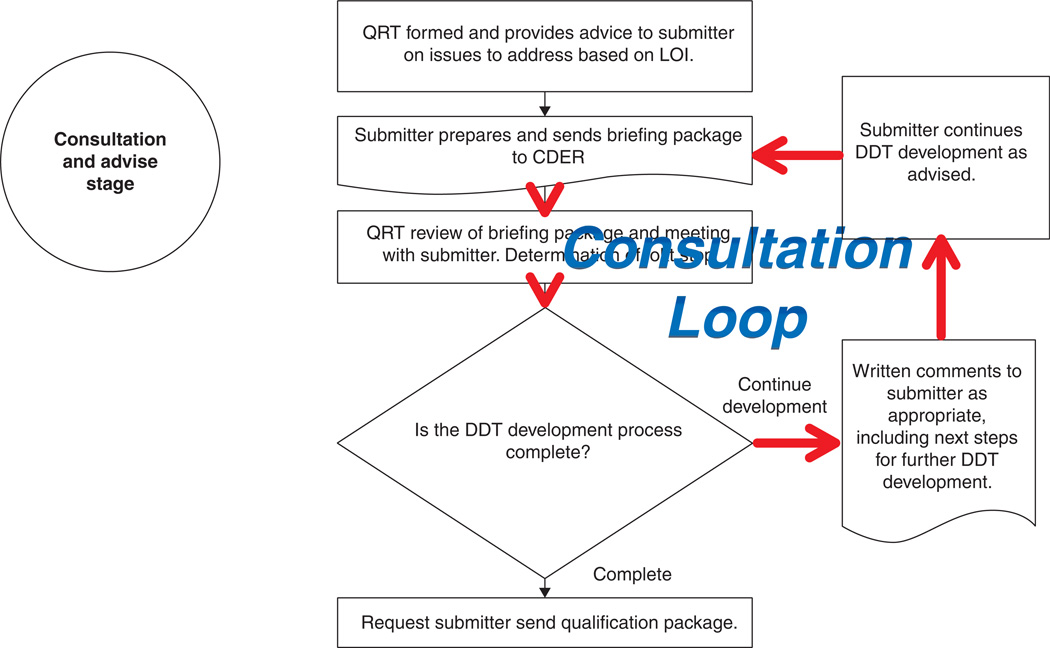
Figure 2. Consultation loop in the Consultation and Advice stage of a biomarker qualification process.
Several stages in current biomarker qualification processes throughout regulatory agencies are vulnerable to prolonged consultation cycles where applicants and reviewers go through multiple cycles of discussion about context of use and evidentiary standards for biomarker qualification.