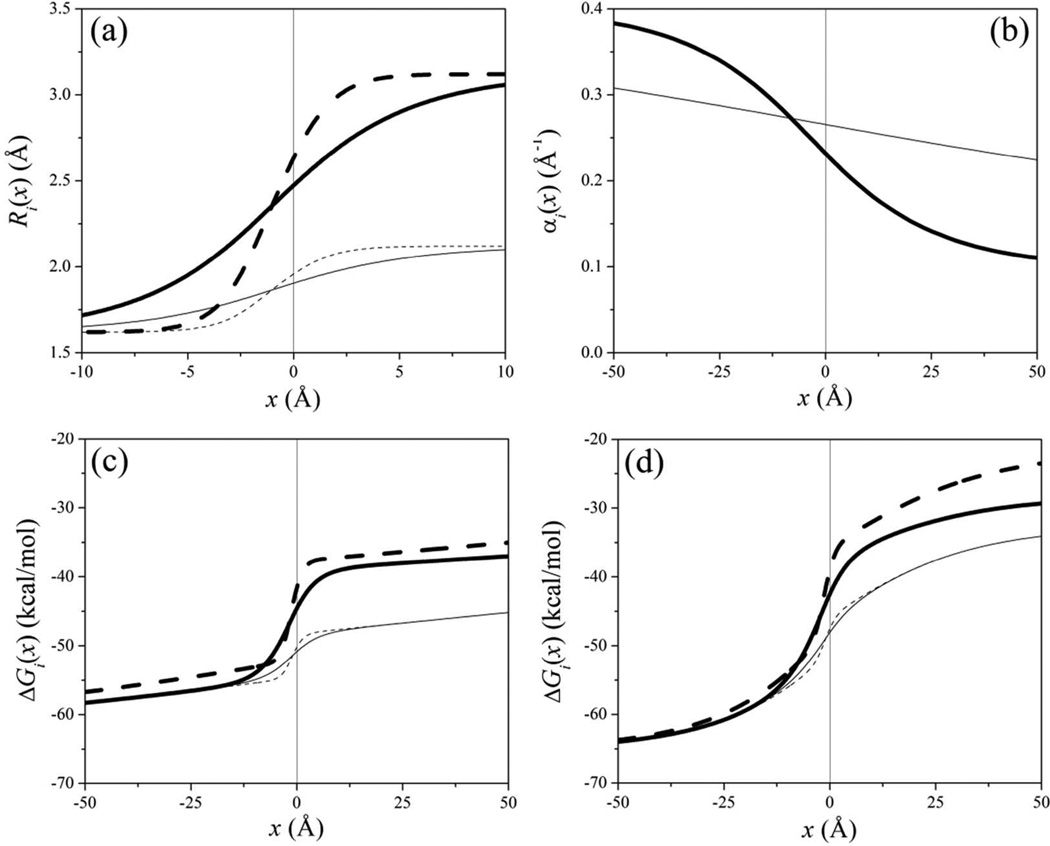
Figure 1.
Behavior of (a) the effective radius R, (b) the screening parameter α, and (c, d) the self-energy ΔG of a point charge q = +1 close to a planar aqueous interface, as described by the SCP continuum solvent model [Eqs. (1–4)]. The interface is located at x = 0, with water filling the space x < 0 and an idealized protein occupying the region x > 0. (a) From Eq. (4), using a = Rw + 1.5 Å (thick lines) and a = Rw + 0.5 Å (thin), for τ = 3.125 Å (solid) and τ = 1.0 Å (dashed). The parameter a determines the total change in effective radius between bulk water (x → −∞) and bulk protein (complete dehydration, x → ∞); τ determines the rate of change as the particle crosses the interface. (b) From Eq. (2), using σ = 15 Å (thick); σ = 75 Å (thin). (c) From Eq. (1) with σ = 75 Å and effective radii plotted in panel (a). (d) Same as in (c), using σ = 15 Å. The protein, which determines the planar aqueous interface, was modeled as two superimposed three-dimensional cubic lattices, one representing the positions of Cα atoms in Eq. (2), with a side length of 7 Å; and the other one representing the position of all atoms in Eq. (4), with a side length of 2 Å (assuming an average volume of ~180 Å3 per amino acid,16 and ~20 atoms per amino acid).