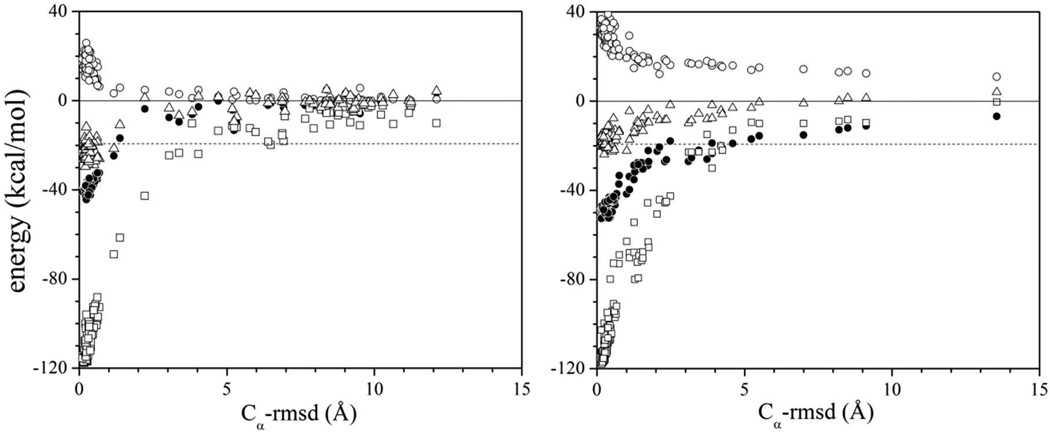
Figure 2.
Non-bonded energy decomposition of the barnase-barstar complex during dissociation by heating, calculated with the SCP continuum solvent model as implemented in the CHARMM program (version c35b4), with (a) and without (b) long-range water-exclusion effects: protein-protein van der Waals energy (squares), electrostatic interaction energy [black circles; first term in Eq. (1)], and self-energy [open circles; second term in Eq. (1)]. The total electrostatic energy Ee [Eq. (1)] of the system is also shown (triangles) and determines the dissociation enthalpy. The reaction coordinate is the Cα-rmsd with respect to the crystal structure of the complex (PDB 1brs). The limits σ → ∞ and σ’ → ∞ in Eqs. (2) and (3) lead to over-stabilization of the complex by ~3.5 kcal/mol. Optimized values σ = 59 Å and σ’ = 37 Å lead to a dissociation energy equal to the measured ΔHb = 19.3 kcal/mol of the complex.