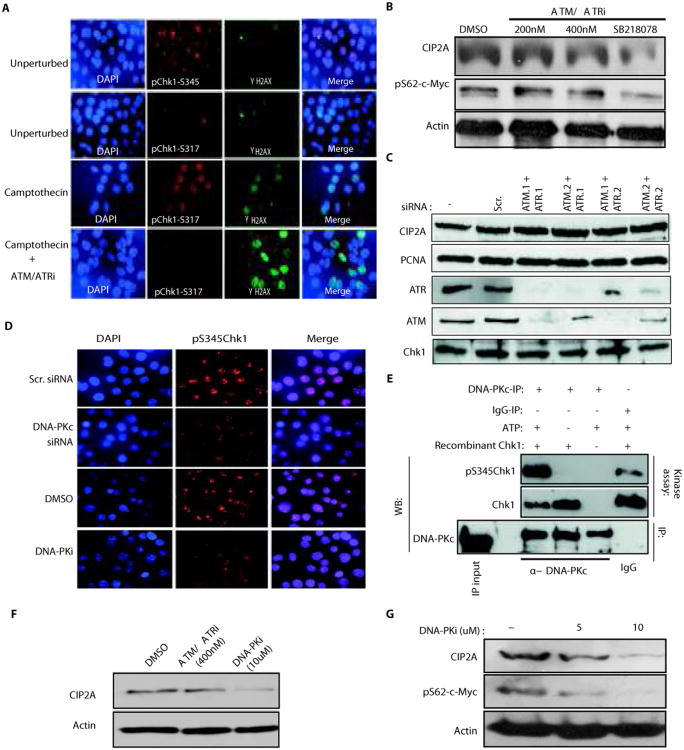
Figure 6.
DNA-PKc acts as an upstream mediator of Chk1 serine345 phosphorylation and CIP2A expression in unperturbed cancer cells. A, Immunoflourescent stainings of unperturbed and Camptothecin (400nM for 24h) and/or ATM-ATR inhibitor (400 nM for 24h) treated AGS cells with indicated antibodies and DAPI. γ-H2AX staining was used to demonstrate Camptothecin-induced double-stranded DNA breaks. B, Western blot analysis of CIP2A and phosphoserine62 MYC expression levels in AGS cells treated either with indicated concentrations of ATM/ATR inhibitor (ATM/ATRi) or with Chk1 inhibitor SB218078(1uM) for 48 h. C, Expression of CIP2A, PCNA, ATM, ATR and Chk1 proteins in MKN-28 cells co-transfected with two independent siRNAs targeting both ATM and ATR, 72h post-transfection. D, Immunoflourescent stainings of AGS cells with DAPI and phospho Chk1-Serine-345 antibody after either transfection of DNA-PK targeting siRNA or treatment with DNA-PK inhibitor (DNA-PKi; 10uM for 48h). E, DNA-PKc immunoprecipitated from exponentially growing MKN-28 cells was used in in-vitro kinase assay with recombinant Chk1 protein as a substrate. Chk1 protein amounts and serine345 phosphorylation was studied form kinase reaction by western blotting. F, CIP2A protein expression in AGS cells treated for 48h with ATM/ATR inhibitor(400nM) or DNA-PK inhibitor(10uM). G, CIP2A protein expression and phospho-MYC-serine62 levels in MKN-28 cells treated for 48h with DNA-PK inhibitor at indicated concentrations. Shown are representative results from two independent experiments.