Figure 1.
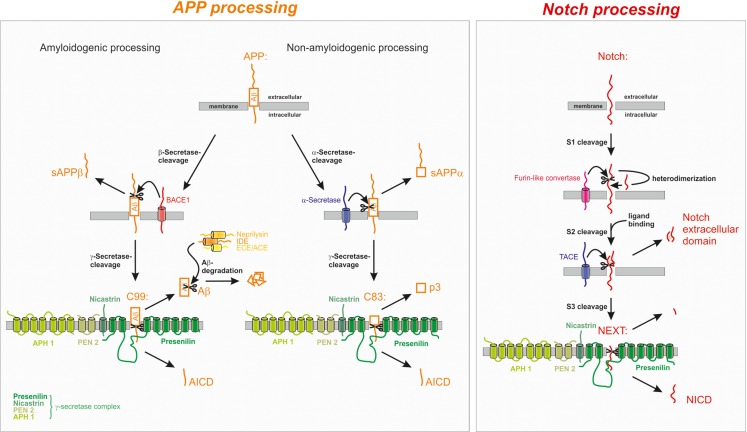
Proteolytic processing of the amyloid precursor protein (APP) and Notch. APP processing: in the amyloidogenic processing pathway, APP is cleaved by the β-secretase BACE1 generating C99 which is further cleaved to the Amyloid-β peptide (Aβ). The non-amyloidogenic processing pathway is initiated by α-secretase cleavage of APP within the Aβ domain, thus precluding the generation of Aβ. α- and β-secretase cleavage releases the soluble forms of APP, sAPPα, and sAPPβ, respectively, into the extracellular space. The remaining membrane-bound C-terminal fragments C83 and C99 are further processed by the γ-secretase complex leading to the generation of the non-toxic p3 from C83 or of the amyloidogenic Aβ peptide from C99. Aβ is rapidly degraded by several enzymes, for example neprilysin (NEP), insulin-degrading enzyme (IDE), endothelin-converting enzyme (ECE), and angiotensin converting enzyme (ACE). In both processing pathways the APP intracellular domain (AICD) is released into the cytosol. Notch processing: after maturation S1 cleavage of the Notch receptor precursor by a furin-like convertase, Notch is processed similarly to APP. Ligand binding triggers the S2 cleavage by the α-secretase TACE/ADAM17 leading to the release of the Notch extracellular domain into the extracellular space. The remaining membrane-bound fragment Notch extracellular truncation (NEXT) is further processed by the γ-secretase complex resulting in the release of the Notch intracellular domain (NICD) from the membrane.