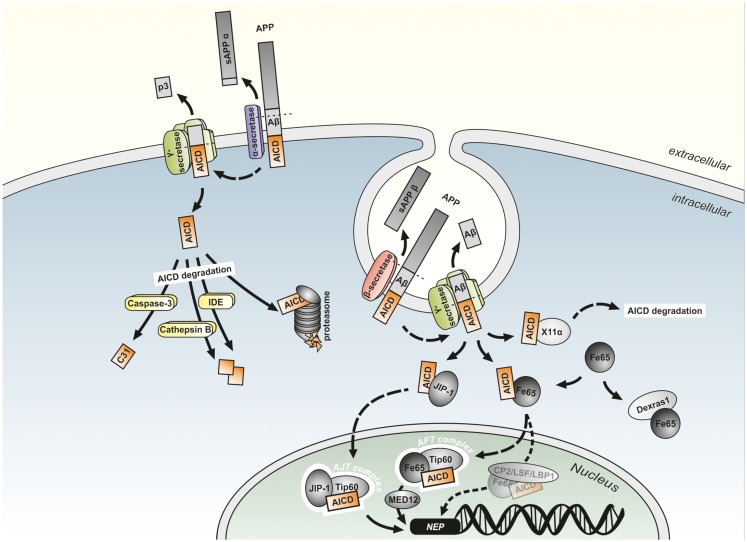
Figure 4.
Model of potential mechanism of AICD-mediated gene regulation. The two different APP cleavage pathways have been shown to occur in distinct subcellular localizations. While the non-amyloidogenic pathway by α- and γ-secretase cleavage takes place at the plasma membrane, the amyloidogenic APP processing is discussed to take mainly place in endosomes. The APP intracellular domain (AICD) generated by non-amyloidogenic APP processing is rapidly degraded by, e.g., the proteasome, insulin-degrading enzyme (IDE), Cathepsin B, and Caspase-3 into smaller fragments. In contrast, AICD generated by amyloidogenic APP processing can be stabilized by binding to Fe65 or JIP-1 and translocated to the nucleus, where the gene regulatory AFT (AICD, Fe65, Tip60) or AJT (AICD, JIP-1, Tip60) complexes are formed. Alternatively to Tip60, the transcription factor CP2/LSF/LBP1 is hypothesized to interact with Fe65 and activate AICD-mediated gene transcription. Binding to the MED12 protein links these complexes to the RNA polymerase transcription apparatus. Additionally binding of AICD to MINT1/X11α or the interaction of Dexras1 with the PTB2 domain of Fe65 results in an inhibition of Fe65/AICD induced transcription.