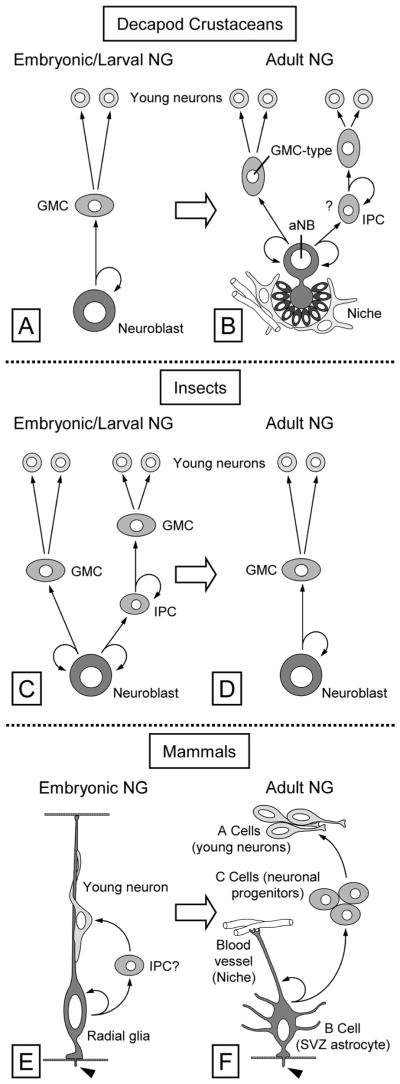
Figure 12.
Schematic comparison of neural stem cells and cell lineages maintaining embryonic/larval and adult neurogenesis in the brain of decapod crustaceans (A,B), insects (C,D), and mammals (E,F). A: Embryonic and larval neurogenesis in crustaceans is based on large, spherical neuroblasts, which through a series of asymmetric divisions self-renew and generate ganglion mother cells (GMC) into the interior of the body. Each GMC divides symmetrically once and generates two immature neurons. B: Adult neurogenesis in decapod crustaceans is maintained by large, hourglass-shaped adult neuroblasts (aNB), which are closely associated with a stem cell niche composed of clump-forming cells, glial cells, and an attached arteriole. Through a series of asymmetric divisions the aNB self-renews and ultimately gives rise to GMC-type neuronal progenitor cells, each of which divides symmetrically once and generates two immature neurons (left lineage). We hypothesize that the daughter cells produced by the aNB divisions likely act as transient-amplifying intermediate progenitor cells (IPC), each of which produces multiple GMC-type neuronal progenitors (right lineage). C: Embryonic and larval neurogenesis in insects is mostly as in crustaceans and is based on “canonical” neuroblasts (left lineage). Some embryonic and larval neuroblast lineages contain transit-amplifying intermediate progenitor cells (IPC) that through asymmetric divisions self-renew and generate GMCs (right lineage; modified from Bello et al., 2008). D: Adult neurogenesis in insects appears to be maintained by “canonical” neuroblasts and their embryo-typical lineage. E: In embryonic neurogenesis of mammals, radial glial cells serve as neural stem cells. Through asymmetric divisions they self-renew and ultimately generate daughter cells that, after migrating along the radial glial process, mature into neurons. The generation of neurons could be direct or through transit-amplifying intermediate progenitor cells (IPC?). One hallmark feature of radial glia cells is to have a primary cilium reaching into the ventricle (arrowhead). Modified from Alvarez-Buylla et al. (2001). F: Adult neurogenesis in the SVZ of mammals is maintained by type B cells, which have an astrocytic morphology and possess a primary cilium (arrowhead) reaching into the ventricle. Type B cells are slowly cycling neural stem cells, which through asymmetric divisions self-renew and generate rapidly dividing neuronal progenitor cells (C cells). C cells give rise to immature neurons (A cells). Type B cells are associated with morphologically complex stem cell niches, one element of which are blood vessels directly contacted by type B cell processes. Modified from Kriegstein and Alvarez-Buylla (2009).