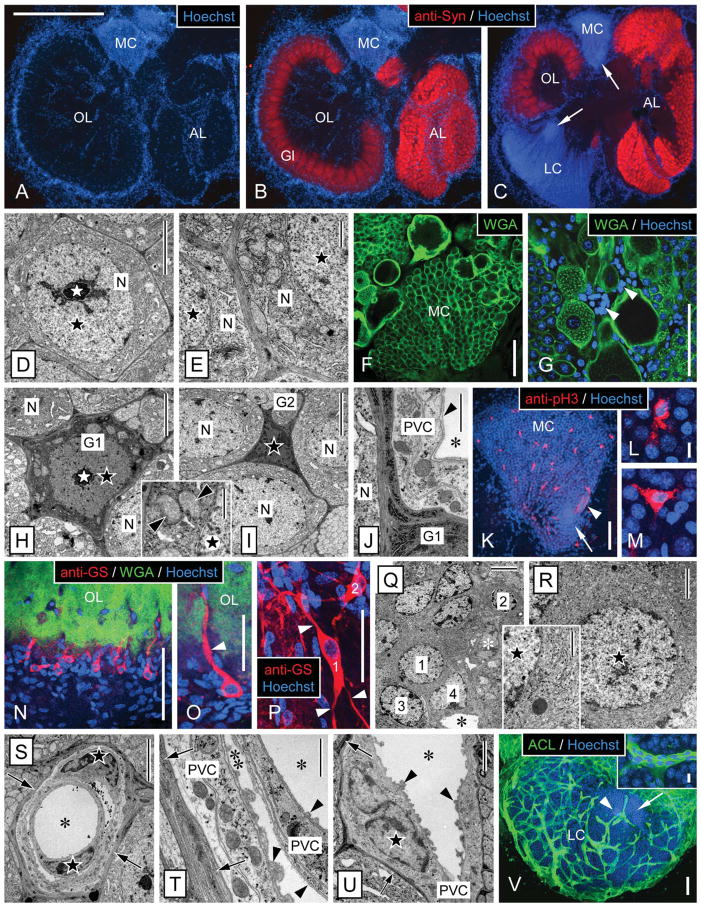
Figure 3.
Morphology of the olfactory deutocerebrum of P. argus and identification of its major cell types by TEM and selective markers. A–C: Main compartments of the olfactory deutocerebrum visualized by double labeling with anti-Syn and Hoechst 33258. Anti-Syn labeled synaptic areas within neuropils: the cortex of columnar glomeruli (Gl) of the olfactory lobe (OL) and the small spherical glomeruli of the accessory lobe (AL). Labeling with the nuclear marker Hoechst 33258 delineated the two large clusters of neuronal somata: the medial soma cluster (MC) and the lateral soma cluster (LC) and in addition numerous cells at the rim and in the center of the OL and AL. A,B: Horizontal section through the central aspect of the olfactory deutocerebrum with the OL at its largest extension. C: Horizontal section through the ventral aspect of the olfactory deutocerebrum with the AL at its largest extension. Within the LC and MC, the proliferation zones (arrows) located at the interior surface of the respective soma cluster can be distinguished based on their higher nuclear density. D–G: Identification of neurons by TEM and labeling with WGA. D: Soma of mature neuron (N) in the LC. The soma has a regular, slightly polyhedral shape and contains an almost spherical nucleus (black star) with a centrally located nucleolus (white star). E: Cytoplasm of neuronal somata (N). A thick layer of cytoplasm surrounds the nucleus (black stars), and it contains numerous mitochondria and open ER cisternae as well as some Golgi apparatuses. F,G: Distribution of WGA labeling in the MC. F: In all neuronal somata, the cell membrane is distinctly labeled, and, in some large neuronal somata, the cytoplasm is also labeled in a punctate pattern. G: Double labeling with WGA and Hoechst 33258 revealed that numerous WGA− cells (arrowheads) most likely representing glial cells and perivascular cells surround large WGA+ neuronal somata. This labeling pattern establishes WGA as neuron-selective marker. H–M: Identification of cell body glia by TEM and labeling with anti-pH3. H: Type 1 cell body glia. The soma is relatively large (in the same size range as neuronal somata) and has an astrocyte-like, multipolar shape because of several processes extending between adjacent neuronal somata (N). It contains a thick layer of cytoplasm with numerous mitochondria (inset, arrowheads) surrounding a regularly shaped nucleus (black star) with a central nucleolus (white star). Cyto- and nucleoplasm are distinctly more electron dense than in neuronal somata. I: Type 2 cell body glia. The soma is small, of irregular shape, and almost completely filled by the irregularly shaped nucleus (black star). As in type 1 cell body glia, the soma extends several processes between adjacent neuronal somata (N) and cyto- and nucleoplasm are electron dense. J: Processes of cell body glia (G1) are characterized by electron-dense cytoplasm containing very electron-dense and robust cisternae of rough ER. Several layers of processes separate a neuronal soma (N) from perivascular cell processes (PVC) forming the wall of an adjacent arteriole (asterisk, lumen of arteriole; arrowhead, basal lamina). K–M: Double labeling with anti-pH3 and Hoechst 33258 in the medial soma cluster (MC). Anti-pH3 selectively labeled cells that based on size, distribution, and multipolar morphology (L,M) are identified as cell body glia (arrow, proliferation zone; arrowhead, clump of cells). N–R: Identification of type 1 neuropil glia by labeling with anti-GS and correlative TEM. N,O: Triple labeling with anti-GS, WGA, and Hoechst 33258 revealed a population of type 1 neuropil glia at the edge of the olfactory lobe (OL). Most of these cells are unipolar and extend one major process (arrowhead) into the WGA+ OL neuropil usually running along a WGA− arteriole. Note that GS+ type 1 neuropil glia represent only a small fraction of the cells located at the edge of the OL neuropil whose nuclei are visualized by Hoechst 33258. P: Double labeling with anti-GS and Hoechst 33258 revealed multipolar (arrowheads) type 1 neuropil glia (1, 2) at the edge of the median protocerebral neuropil. Q: TEM revealed that the cell layer surrounding the OL neuropil is composed of diverse types of putative glial cells (1, 2, 3, 4) that among each other differ distinctly in nuclear morphology and/or cytoplasmic composition (white asterisk, nucleus of perivascular cell; black asterisk, lumen of arteriole). R: Putative glial cell at the edge of the OL neuropil (1 in Q) that corresponds to GS+ type 1 neuropil glia in having an almost spherical nucleus, a thick layer of cytoplasm (inset: note delicate cisternae of rough ER), and unipolar morphology. S–V: Identification of perivascular cells by TEM and labeling with ACL. S–U: By TEM, arterioles are unequivocally identified as extracellular empty spaces representing the arteriole lumen (asterisks) enclosed by processes of perivascular cells (PVC) that are electron lucent and have a distinct cytoplasmic composition. The nuclei of perivascular cells (black stars) have an irregular, flattened shape and contain peripheral heterochromatin. Most arterioles are simple vessels (U), but some are complex, being composed of a vessel within a vessel (S,T), creating an inner (single asterisk) and an outer arteriole lumen (double asterisk). A basal lamina (arrowheads) composed of unstructured, flocculent material overlays all luminal surfaces of perivascular cell processes. Note that perivascular cells are separated from other tissue elements by an electron-dense layer (arrows) composed of processes of cell body glia. V: Double labeling with ACL and Hoechst 33258 revealed that, in the lateral soma cluster (LC), ACL intensely labels the net-like system of arterioles. Note that the proliferation zone (arrow) is less permeated by arterioles than the periphery of the LC and that the clump of cells (arrowhead) is attached to an arteriole. Inset: Intense and uniform ACL labeling distinguishes perivascular cells from surrounding neuronal somata. Scale bar = 1 mm in A (applies to A–C); 5 μm in D,H,I,Q,S; 1 μm in E,J,T, H inset, Q inset; 100 μm in F,G,K,N,O,V; 10 μm in L (applies to L,M); 10 μm in V inset; 50 μm in P; 2 μm in R,U.