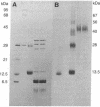
Abstract
A stoichiometric model of the photosynthetic unit of Ectothiorhodospira halochloris has been obtained by means of scanning transmission electron microscope mass determination and mass mapping in conjunction with polyacrylamide gel electrophoresis. One reaction center, consisting of four single polypeptides, including one cytochrome, is surrounded by six identical light-harvesting complexes, each containing three polypeptides with 2:2:2 stoichiometry. This stoichiometric model was incorporated into the three-dimensional structure of the photosynthetic unit as derived from surface relief reconstructions of the two surfaces of shadowed membranes. The reaction center protrudes substantially from both membrane surfaces and has the cytochrome attached to the periplasmic face in a noncentrosymmetric fashion. The reaction center may assume various orientations within the photosynthetic complexes.
Keywords: mass mapping, scanning transmission electron microscopy, image processing, three-dimensional reconstruction, bacterial photosynthetic membrane
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunisholz R. A., Jay F., Suter F., Zuber H. The light-harvesting polypeptides of Rhodopseudomonas viridis. The complete amino-acid sequences of B1015-alpha, B1015-beta and B1015-gamma. Biol Chem Hoppe Seyler. 1985 Jan;366(1):87–98. doi: 10.1515/bchm3.1985.366.1.87. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Engel A., Baumeister W., Saxton W. O. Mass mapping of a protein complex with the scanning transmission electron microscope. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4050–4054. doi: 10.1073/pnas.79.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. Molecular weight determination by scanning transmission electron microscopy. Ultramicroscopy. 1978;3(3):273–281. doi: 10.1016/s0304-3991(78)80037-0. [DOI] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Multivariate statistical analysis of ribosome electron micrographs. L and R lateral views of the 40 S subunit from HeLa cells. J Mol Biol. 1982 Oct 15;161(1):107–133. doi: 10.1016/0022-2836(82)90281-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto F., Horigome T., Kanbayashi M., Yoshida K., Sugano H. An improved method for separation of low-molecular-weight polypeptides by electrophoresis in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1983 Feb 15;129(1):192–199. doi: 10.1016/0003-2697(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Jay F., Lambillotte M., Mühlethaler K. Localisation of Rhodopseudomonas viridis reaction centre and light harvesting proteins using ferritin-antibody labelling. Eur J Cell Biol. 1983 Mar;30(1):1–8. [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Lottspeich F. The ;heavy' subunit of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the gene, nucleotide and amino acid sequence. EMBO J. 1985 Jul;4(7):1667–1672. doi: 10.1002/j.1460-2075.1985.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. R. Structure of a bacterial photosynthetic membrane. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6415–6419. doi: 10.1073/pnas.76.12.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J. Composition and development of the bacterial photosynthetic apparatus. Subcell Biochem. 1981;8:1–73. doi: 10.1007/978-1-4615-7951-9_1. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Stark W., Kühlbrandt W., Wildhaber I., Wehrli E., Mühlethaler K. The structure of the photoreceptor unit of Rhodopseudomonas viridis. EMBO J. 1984 Apr;3(4):777–783. doi: 10.1002/j.1460-2075.1984.tb01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Cogdell R. J., Seftor R. E., Webster G. D. Further studies on the composition and spectral properties of the photochemical reaction centers of bacteriochlorophyll b-containing bacteria. Biochim Biophys Acta. 1980 Nov 5;593(1):60–75. doi: 10.1016/0005-2728(80)90008-0. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]