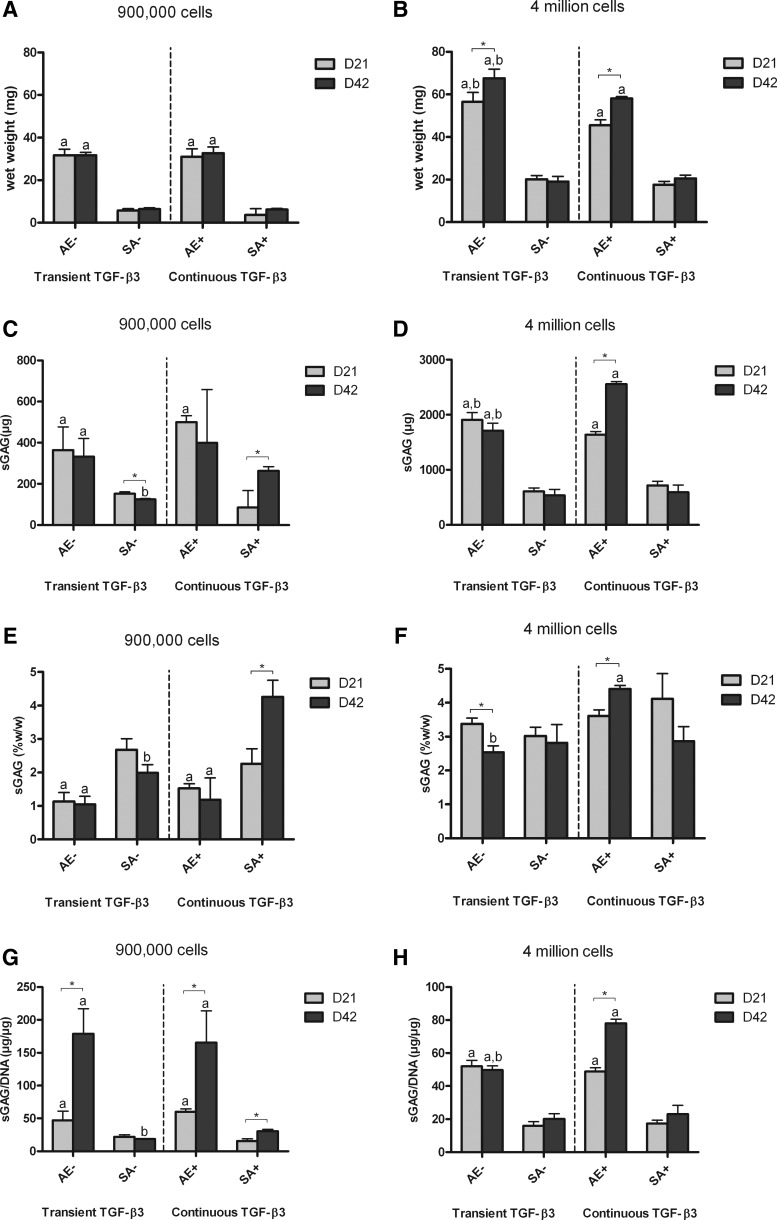
FIG. 2.
Wet weight and sulphated glycosaminoglycan (sGAG) content of agarose encapsulation (AE) and SA constructs for both transient (AE−, SA−) and continuous (AE+, SA+) transforming growth factor (TGF)-β3 supplementation. (A) wet weight for low seeding density constructs (mg); (B) wet weight for high seeding density constructs (mg); (C) sGAG content for low seeding density constructs (μg); (D) sGAG content for high seeding density constructs (μg); (E) sGAG content normalized to wet weight for low seeding density constructs (%w/w); (F) sGAG content normalized to wet weight for high seeding density constructs (%w/w); (G) sGAG content normalized to DNA content for low seeding density constructs (μg/μg); (H) sGAG content normalized to DNA content for high seeding density constructs (μg/μg). ap<0.05 versus SA with same culturing conditions at same time point; bp<0.05 versus continuous TGF-β3 with same scaffold at same time point. *Denotes significant difference with p<0.05.