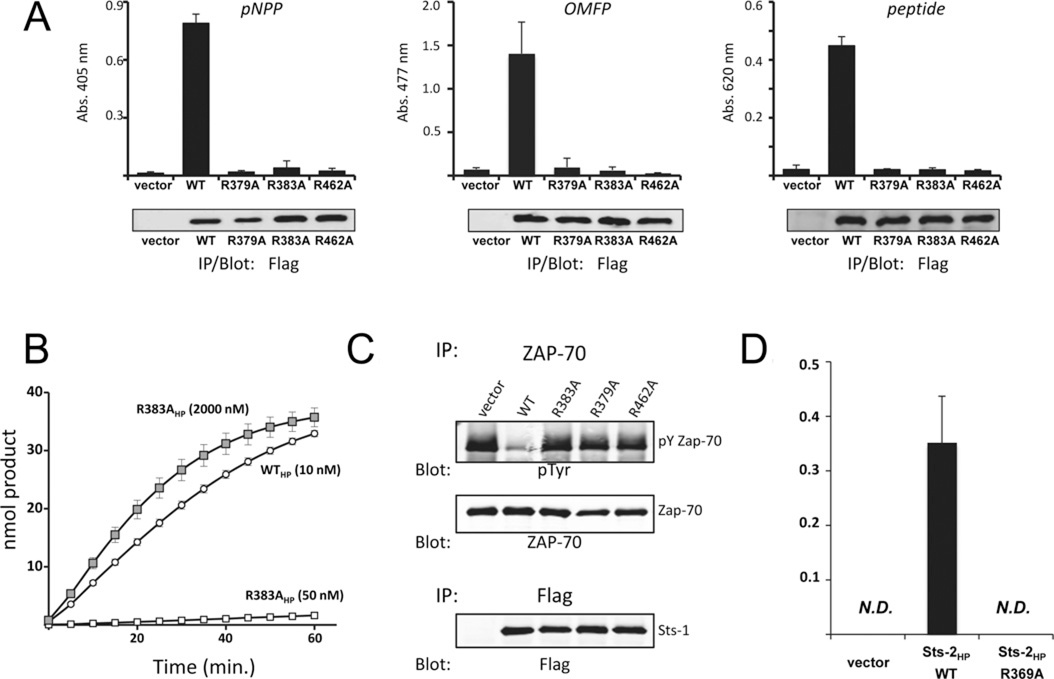
Figure 2. Requirement for Arg383/Arg369 in Sts-1/Sts-2 catalytic activity.
(A) Immune complex phosphatase activity assays of wild-type (WT) Sts-1HP and the indicated mutants using as substrates pNPP (left-hand panel), OMFP (middle panel) or a phospho-tyrosine phosphopeptide (right-hand panel) demonstrate the requirement for Sts-1 Arg383 for catalytic activity. Sts-1 R379A and Sts-1 R462A serve as negative controls. Results are means ± S.D. representative of multiple experiments with two replicates for each condition throughout. Abs., absorbance. (B) Phosphatase activity time course with recombinant wild-type Sts-1HP (WTHP) and Sts-1HP R383A at the indicated concentrations demonstrates impairment of Sts-1HP R383A. The concentration of pNPP was 1 mM. (C) Co-expression of Zap-70 in HEK-293 cells with the indicated Sts-1 constructs indicates Sts-1 R383A is unable to dephosphorylate full-length Zap-70. The levels of Zap-70 phosphorylation were assessed by anti-phospho-tyrosine (pY/pTyr) immunoblotting. The loading controls are indicated. (D) OMFP hydrolysis activity of Sts-2 R369A is impaired relative with wild-type Sts-2, as demonstrated in an immune complex phosphatase assay. Results are means ± S.D. IP, immunoprecipitation; N.D., not determined.