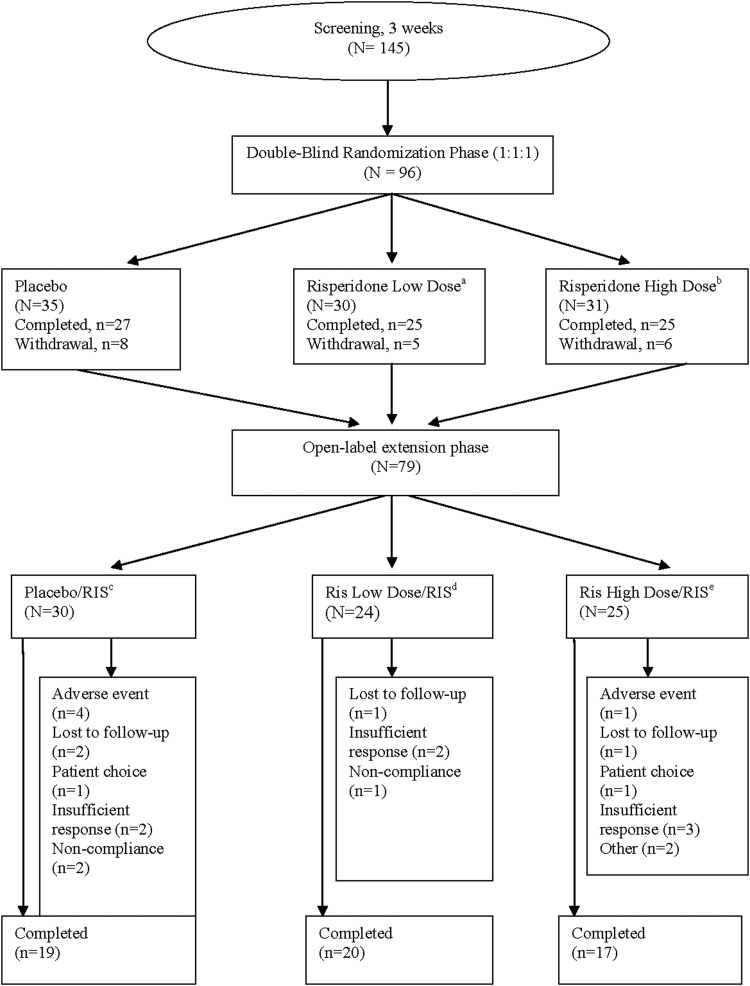
FIG. 1.
Study design and patient disposition. a0.125 mg/day (patients weighing 20 kg to <45 kg). b0.175 mg/day (patients weighing≥45 kg). cPatients randomly assigned to placebo during double-blind (DB) phase who continued into open-label extension (OLE) and received risperidone. dPatients randomly assigned to risperidone low dose in DB phase and receiving risperidone (as per weight class) in OLE phase. ePatients randomly assigned to risperidone high dose in DB phase and receiving risperidone (as per weight class) in OLE phase.