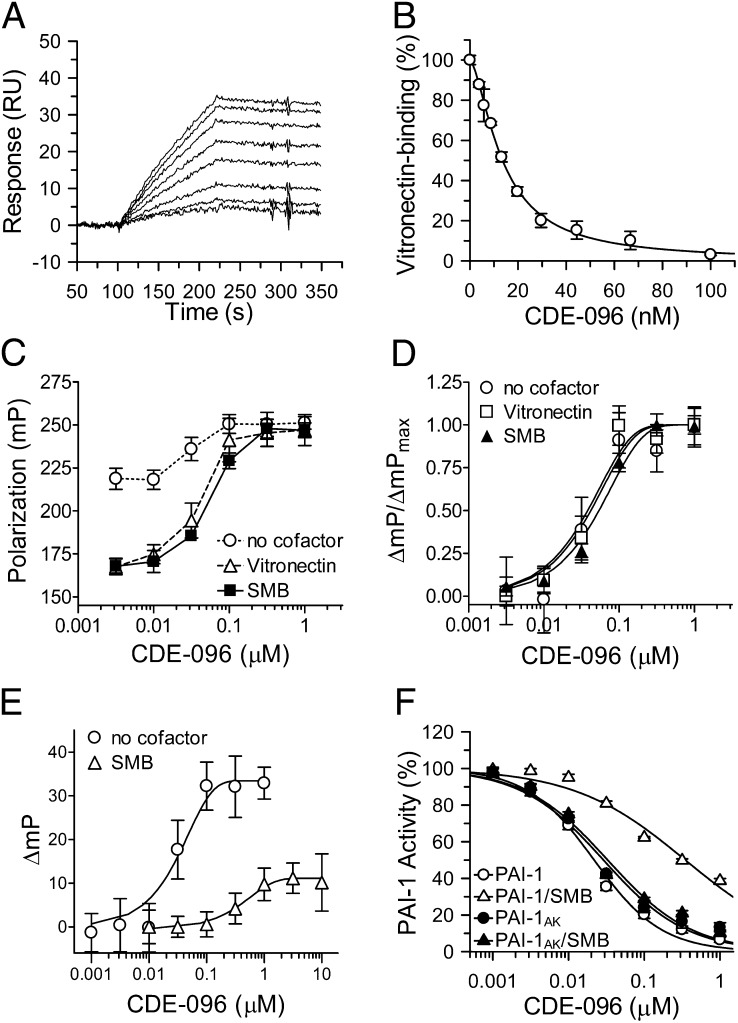
Fig. 3.
CDE-096 and vitronection reciprocally decrease binding for one another to PAI-1. (A) Real-time binding sensograms of PAI-1 to immobilized vitronectin, in the presence of CDE-096 (0, 3.9, 5.8, 8.8, 12.2, 19.8, 29.6, 44.4, 66.7, and 100 nM) with the top-most tracing showing no CDE-096 and the bottom-most tracing showing PAI-1 treated with 100 nM of CDE-096. (B) From the data in A, the initial association phase of PAI-1 binding to vitronectin in the presences of each concentration of CDE-096 was linearly fit and the slopes plotted against the CDE-096 concentration. (C) FP of 5 nM PAI-1S149C-FL in the presence of increasing concentrations of CDE-096 with and without cofactor. Samples of premixed PAI-1S149C-FL and 0–1μM CDE-096 were further incubated with either no cofactor, 100 nM vitronectin, or 100 nM SMB, and the FP signal was measured. (D) Normalization of the FP signal shown in C reveals identical dose dependence of the three samples to CDE-096. (E) Binding of CDE-096 to 5 nM PAI-1S149C-FL with either no cofactor present or after preincubated of PAI-1 with 30 nM SMB, as monitored by FP. (F) Direct binding to SMB is required to protect PAI-1 against inhibition by CDE-096. PAI-1 (open symbols) or a variant of PAI-1 (PAI-1AK) that is unable to bind SMB (closed symbols) was preincubated either alone (○,●) or with 30 nM SMB (△,▲) before the addition of CDE-096 and the determination of PAI-1 inhibitory activity against uPA.