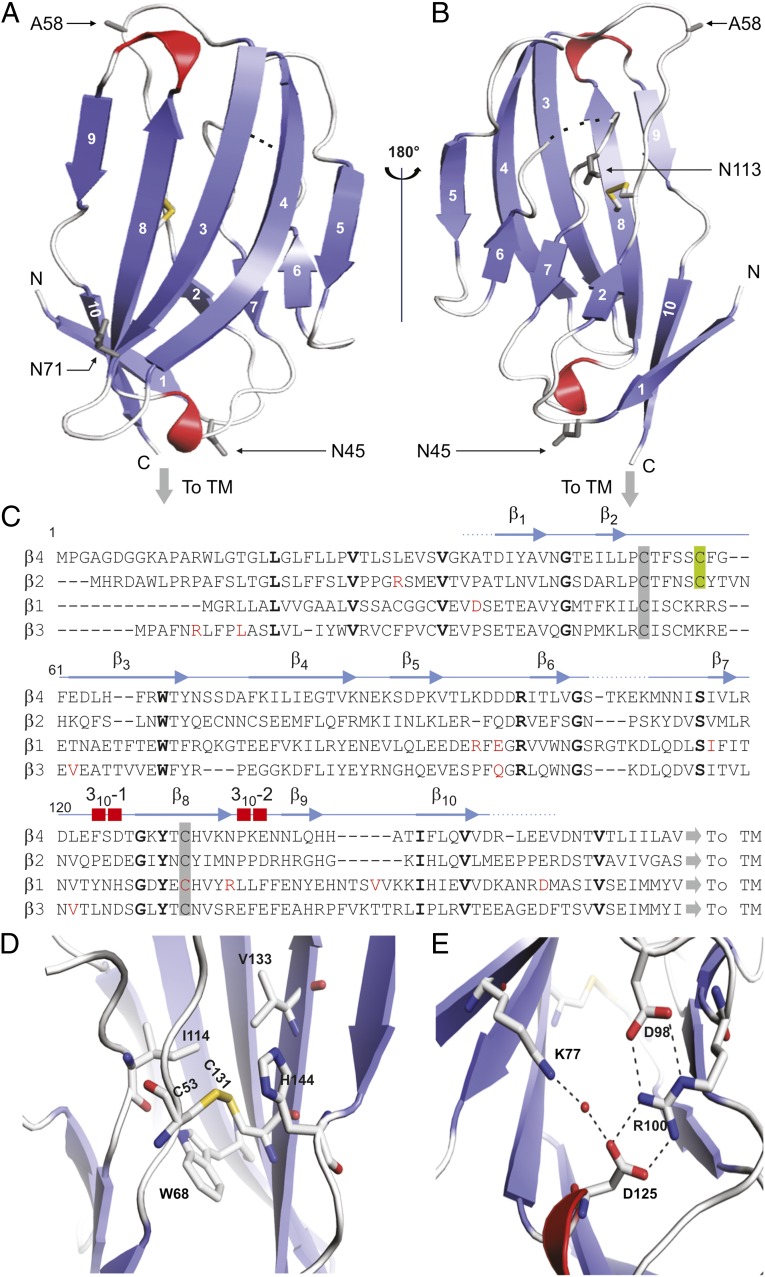
Fig. 2.
Crystal structure of the β4 extracellular domain. (A and B) Two views of the hβ4 extracellular domain, rotated by 180° around a vertical axis. β-Strands are in blue; 310 helices are in red. Dotted lines indicate a flexible region that is invisible in the electron-density map. The conserved cysteine bridge is shown in stick format, with the sulfur atoms colored yellow. The C58A mutation is indicated as A58, and three potential glycosylation sites are shown in as 45Asn (N45), 71Asn (N71), and 113Asn (N113). “To TM” indicates the position at which the single-transmembrane helix starts; N and C specify the N- and C-terminal ends of the structured part of the extracellular domain, respectively. (C) Sequence alignment of the extracellular regions of hβ1–4 with the secondary structure of β4 shown above. Amino acids conserved between β4 and other β-subunits are in bold, and known β-subunit–related disease mutations are in red. Conserved cysteines are highlighted by a gray background and the C58A locus by a green background. (D) Close-up view of the disulfide bond showing the nearby conserved hydrophobic core. (E) Close-up view of the ion-pair network formed by hydrogen bonds between 98Asp and 100Arg, which forms two additional bonds with 125Asp as well as a water-mediated hydrogen bond between 77Lys and 125Asp.