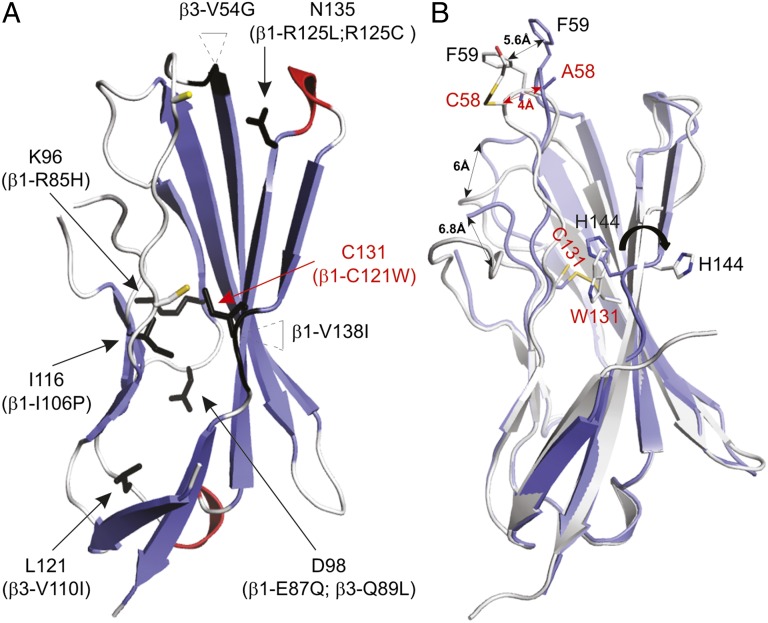
Fig. 5.
Disease-related mutations mapped onto the β4 extracellular domain structure and crystal structure of the β4 C131W variant. (A) Arrows indicate the positions of known β1 and β3 disease mutations (summarized in Table S2) mapped onto the β4 extracellular domain. The corresponding residue substitutions are shown in parentheses, and the positions of 53Cys and 58Cys are indicated by a yellow stick for reference purposes. Two mutations occur within inserted regions, and the main chains of amino acids next to these insertions are also colored black. (B) The crystal structure of the C131W mutant (white) superimposed onto the WT β4 crystal structure (blue). Shifts in the positions of several regions are indicated by double-headed arrows, and select side chains are shown for reference purposes. 58Cys in the C131W structure appears to have a 2-ME molecule attached to it, further highlighting its reactivity. In both A and B, mutants discussed in this work are displayed in red.