Significance
Silica-shelled diatoms dominate marine phytoplankton blooms and play a key role in ocean ecology and the global carbon cycle. We show how differences in ecological traits of dominant Southern Ocean diatom species, observed during the in situ European Iron Fertilization Experiment (EIFEX), can influence ocean carbon and silicon cycles. We argue that the ecology of thick-shelled diatom species, selected for by heavy copepod grazing, sequesters silicon relative to other nutrients in the deep Southern Ocean and underlying sediments to the detriment of diatom growth elsewhere. This evolutionary arms race provides a framework to link ecology with biogeochemistry of the ocean.
Keywords: evolutionary arms race, top-down control, geo-engineering
Abstract
Diatoms of the iron-replete continental margins and North Atlantic are key exporters of organic carbon. In contrast, diatoms of the iron-limited Antarctic Circumpolar Current sequester silicon, but comparatively little carbon, in the underlying deep ocean and sediments. Because the Southern Ocean is the major hub of oceanic nutrient distribution, selective silicon sequestration there limits diatom blooms elsewhere and consequently the biotic carbon sequestration potential of the entire ocean. We investigated this paradox in an in situ iron fertilization experiment by comparing accumulation and sinking of diatom populations inside and outside the iron-fertilized patch over 5 wk. A bloom comprising various thin- and thick-shelled diatom species developed inside the patch despite the presence of large grazer populations. After the third week, most of the thinner-shelled diatom species underwent mass mortality, formed large, mucous aggregates, and sank out en masse (carbon sinkers). In contrast, thicker-shelled species, in particular Fragilariopsis kerguelensis, persisted in the surface layers, sank mainly empty shells continuously, and reduced silicate concentrations to similar levels both inside and outside the patch (silica sinkers). These patterns imply that thick-shelled, hence grazer-protected, diatom species evolved in response to heavy copepod grazing pressure in the presence of an abundant silicate supply. The ecology of these silica-sinking species decouples silicon and carbon cycles in the iron-limited Southern Ocean, whereas carbon-sinking species, when stimulated by iron fertilization, export more carbon per silicon. Our results suggest that large-scale iron fertilization of the silicate-rich Southern Ocean will not change silicon sequestration but will add carbon to the sinking silica flux.
Diatoms—silica-shelled unicellular phytoplankton—are major exporters of organic carbon from the surface to the deep ocean and sediments and, hence, influence ocean nutrient cycles and atmospheric CO2 levels (1, 2). However, silicate concentrations, for which diatoms have an obligate demand, vary widely over the nutrient-rich regions of the oceans (3). This is largely due to processes decoupling silicon cycling from that of other nutrients and carbon in surface waters of the Antarctic Zone (AZ), the southernmost belt of the Antarctic Circumpolar Current (ACC) (4). Thus, Si concentrations decline across the AZ from >70 mmol Si⋅m−3 in upwelling waters along its southern boundary (the Antarctic Divergence) (5) to <5 mmol Si⋅m−3 along the Antarctic Polar Front (APF) (6). The corresponding decline in nitrate is much smaller, from 30 to 23 mmol N⋅m−3. The resulting Si/N export ratio of 9/1 is much higher than the average diatom Si/N ratio of ∼1/1 (7, 8). The paradox (9) can partly be explained by increasing Si/N ratios with iron deficiency recorded in many species (10–13) in addition to the exceptionally thick frustules of some ACC diatom species (14), which can reach Si/N ratios of >4:1 in Fragilariopsis kerguelensis (15).
A portion of the silica shells (frustules) sinking out of the northward-propagating surface Ekman layer dissolves in the southward-propagating deep water and is returned as Si to the surface in upwelling water along the Antarctic Divergence (1). This vertical recycling loop between surface and deep water supports growth of thick-shelled diatoms in the surface and functions as a global ocean silicon trap in the deep-water column. Another portion, mainly comprising robust frustules of comparatively few species, of which Fragilariopsis kerguelensis and Thalassiothrix antarctica are particularly common (16–18), is buried as diatom ooze in sediments underlying the iron-limited ACC, which functions as a major global silicon sink (19), accounting for 42–48% of the total marine silica removal (20). In contrast, the sediments underlying productive regions in the ACC, where phytoplankton blooms fertilized by iron input from land masses (21, 22), shelf sediments or dust occur regularly (23), have 10-fold higher carbon contents (>2% C of dry matter) (24), and are dominated by spores of the ubiquitous diatom genus Chaetoceros (25, 26).
The massive removal of silicon relative to nitrogen from the surface layer by the diatoms of the low-productive, iron-limited AZ ecosystem implies that, in addition to the heavy silicification of ACC diatoms (14), a significant proportion of their nitrogen demand will have to be provided by a highly efficient recycling system in the surface layer (27). In contrast to phytoplankton, copepod-dominated zooplankton stocks of high-nutrient, low-chlorophyll (HNLC) regions of the oceans are comparatively large (28, 29). In fact, their grazing pressure was considered to control phytoplankton biomass in HNLC regions before iron limitation was firmly established (30). It has since been hypothesized that copepod feeding and defecation are part of the recycling system (31) and that the phytoplankton species that accumulate biomass in the face of heavy grazing pressure will have evolved some form of defense (32), most likely the heavily silicified frustules characteristic of ACC diatoms (14).
As the Si-depleted northern ACC surface layer is the major source of nutrients upwelling in low latitudes (4), Si retention in the ACC constrains diatoms from forming blooms over large, nutrient-rich areas of the ocean (3) with far-reaching repercussions on food webs and ocean carbon sequestration. A better understanding of the deep water silicon trap and sedimentary sink is necessary to explain functioning of the glacial Southern Ocean (33) and its impact on CO2 drawdown, but also to predict the response of Southern Ocean biota to large-scale and long-term artificial iron fertilization (34). Ocean iron fertilization experiments provide the necessary conditions for the quantitative investigation of these mechanisms because they simulate the effect of natural iron input on pelagic ecosystems with their full complement of grazers and pathogens (34).
Results and Discussion
The Experiment.
The European Iron Fertilization Experiment (EIFEX) was carried out during RV Polarstern cruise ANT-XXI/3 in late austral summer [11 February (day −1) to 20 March (day 36) 2004] in the 60-km diameter, clockwise rotating core of a mesoscale, vertically coherent eddy extending to the seafloor at ∼3,700-m depth and enclosed in a meander of the APF centered at 49°S, 2°E in the Atlantic sector of the Southern Ocean (35). The circular patch of initially 167 km2 had spread to 798 km2 by day 19 and completed four rotations within the eddy by day 36. In-stations were placed in the least diluted region (the hot spot) of the patch, hence sampled the same water mass throughout. Out-stations were always located within the closed core of the eddy, well away from the patch (35). Vertical coherence of the deep-water column with the overlying surface layer was confirmed by the trajectories of four autonomous APEX floats positioned between 200- and 1,000-m depth, as well as two independent models based on hydrographical profiles to the seafloor and on satellite altimetry (35).
Here, we show that the strong biogeochemical response to iron addition (35) was closely linked to temporal changes in the populations of dominant diatom species in surface, subsurface, and deeper layers. Standing stocks (carbon per square meter) are derived from trapezoidal integration of measurements carried out on six to nine discrete water samples taken from standard depths at 10- to 20-m intervals in the 100-m mixed layer (35), 50-m intervals in the 200- to 350-m depth layer, and larger intervals for the deep-water column down to the seafloor. In addition to full (living) cells, intact empty and broken diatom frustules, empty and damaged tintinnid loricae and copepod fecal pellets were counted under a light microscope to assess species-specific diatom and tintinnid mortality and grazing by the copepod assemblage. Empty frustules and loricae can be caused by (i) cell death, (ii) sexual reproduction, (iii) viral infection, or (iv) protozoan and metazoan grazing, whereas broken frustules and damaged loricae are due to handling by copepod mandibles (36). Stocks of particulate organic carbon (POC) and nitrogen (PON), chlorophyll a (Chl a), and biogenic silica (BSi) were highly correlated (P << 0.05) with total plankton, phytoplankton, and diatom carbon (DC), respectively, as estimated from organism counts (Figs. 1 and 2B).
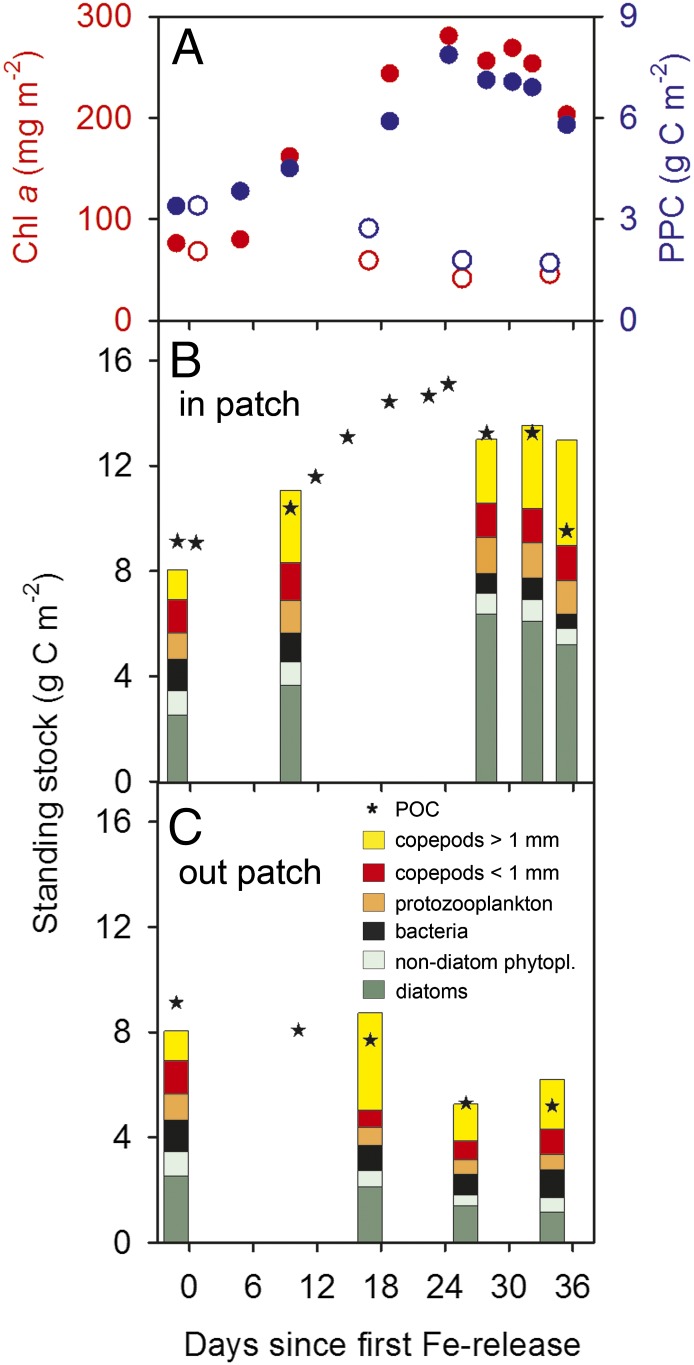
Fig. 1.
Chl a, phytoplankton carbon (PPC), and integrated stocks of plankton composition in the 100-m surface layer inside and outside the fertilized patch. (A) PPC calculated from biovolume data (blue circles) and Chl a stocks (red circles). The full and open circles are for inside and outside the fertilized patch, respectively. Total plankton carbon (bars) and particulate organic carbon (POC) (stars) stocks inside (B) and outside (C) the patch with the contributions of diatoms, nondiatom phytoplankton (largely nanoflagellates: solitary Phaeocystis cells and Prorocentrum spp.), bacteria, protozooplankton (largely heterotrophic dinoflagellates, ciliates, and acantharia), small copepods <1 mm (including nauplii, small copepodites, and Oithona), and large copepodites and adult copepods >1 mm.
Fig. 2.
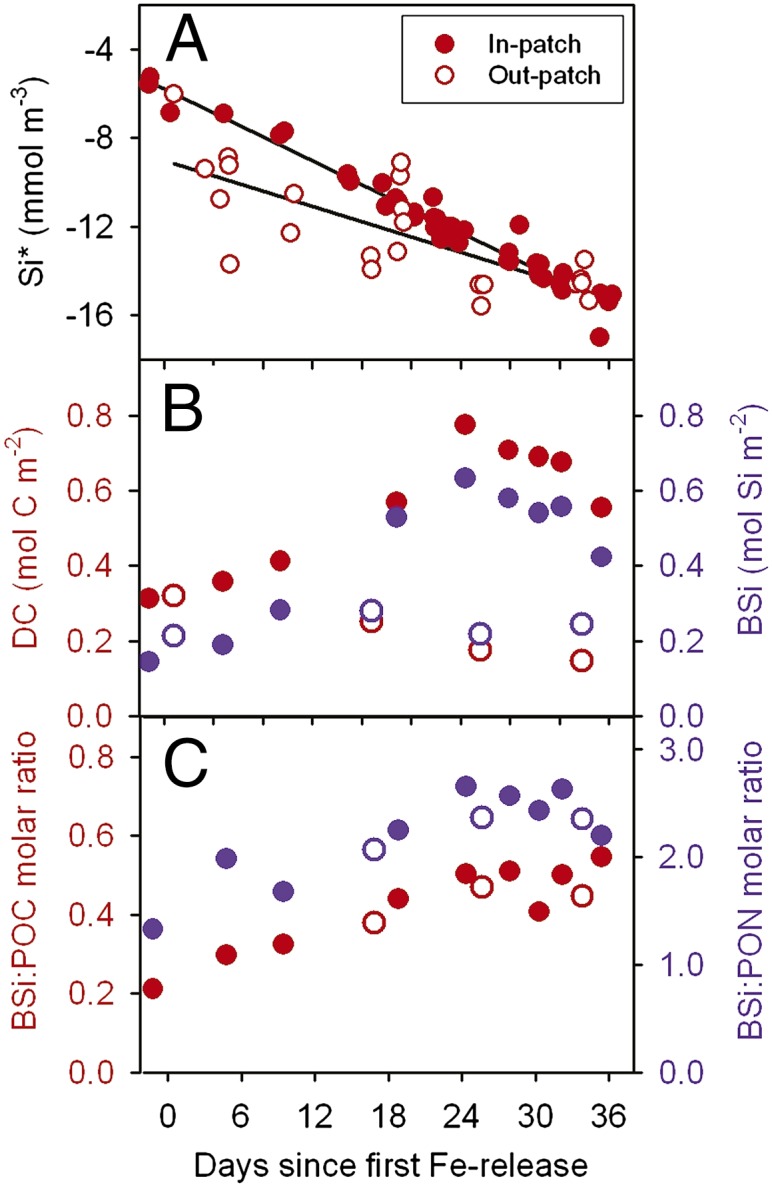
Silicon dynamics in relation to carbon and nitrogen. (A) Uptake of Si inside (full circles) and outside (open circles) the patch expressed as Si* (Si-nitrate concentrations). Because nitrate decreased only slightly, Si* values parallel Si concentrations. The greater scatter in out-patch values is explained by spatial heterogeneity in the core outside the patch (in-patch: r2 = 0.96, P < 0.0001; out-patch: r2 = 0.58, P < 0.0001). (B) Biogenic silica (BSi) (blue) and diatom carbon stocks including equivalent carbon content of empty and broken frustules (DC) (red) for direct comparison with BSi. (C) Ratios of biogenic silica to particulate organic carbon (BSi/POC) (red), and of biogenic silica to particulate organic nitrogen (BSi/PON) (blue). Note similarity in ratios outside and inside the patch.
Plankton Trends.
The initial situation.
During the initial mapping of the eddy, nitrate, phosphate, and silicate concentrations inside the core were 24.5–25 mmol N⋅m−3, 1.7–1.8 mmol P⋅m−3, and 14–19 mmol Si⋅m−3, respectively, which, together with the hydrographical properties, demonstrated that the core originated from the AZ (35). Chl a within the core was patchy, ranging between 0.7 and 1.2 mg⋅m−3, which is twofold to fivefold higher than average AZ concentrations (37). Phytoplankton carbon (PPC) was dominated by large, spiny, heavily silicified (thick-shelled) diatoms typical of the AZ with nanoflagellate species contributing <30%. Heterotrophic biomass exceeded that of autotrophs and comprised bacteria (26%), protozooplankton (22%), and metazooplankton dominated by copepods (52%), including all larval stages (Fig. 1 B and C). The initial small salp population declined during the experiment to negligible biomass (38). 15N and 13C isotope ratios of suspended organic particles indicated that community biomass was primarily based on recycled nitrogen (39).
Trends outside the patch.
Outside the patch, dissolved inorganic carbon (corrected for air–sea exchange), nitrate, and phosphate declined by 1, 0.4, and 0.04 mmol⋅m−3, respectively (35), but silicate decreased considerably by 8 mmol⋅m−3 over 5 wk. This decoupling of silicate and nitrate is reflected in the decline of the already low Si* values [Si – nitrate concentrations (4)] from −6 to −14 mmol⋅m−3 (Fig. 2A). The decrease of Si* cannot be explained by horizontal mixing inside the eddy (35) and only partly by local patchiness (Fig. S1) and thus is largely due to silicate uptake, which must have been matched by an equally high sedimentation rate, as BSi levels in the mixed layer remained stable at 0.2 mol Si⋅m−2 (Fig. 2B). The substantial diatom growth was apparently supported by ammonium remineralized from the dissolved organic nitrogen (DON) pool, which declined linearly from ∼4 to ∼2 mmol N⋅m−3 during the experiment (35). However, after about 3 wk, rising ammonium concentrations (from <0.5 to >0.7 mmol N⋅m−3) suggest that the system switched to net heterotrophy due to iron limitation of autotrophs [low photochemical efficiency (Fv/Fm ratio) of 0.32] compounded by mounting grazing pressure. This scenario is supported by low autotrophic/heterotrophic biomass ratios between 0.4 and 0.5 (Fig. 1C) and by low primary and bacterial production rates (35). Copepods comprised >50% of heterotrophic biomass, but, although fecal pellet stocks increased sevenfold during the 5 wk, the contribution of whole pellets and recognizable remnants to vertical flux was minor as the bulk were fragmented in the surface layer and stocks in the subsurface layer were only a small fraction of surface stocks (Fig. S2). In contrast, the stocks of empty and damaged tintinnid loricae, of similar size to pellet fragments, hence with similar sinking rates, did not differ between layers (Fig. S2), indicating that sinking and not destruction was the major loss term here. We conclude that copepods will have played a key role in maintaining the silica-sinking, nitrogen-recycling ecosystem.
Trends inside the patch.
Inside the fertilized patch, stocks of Chl a, POC, and BSi increased linearly following iron addition due to the growth of various large diatom species whose biomass accounted for 97% of the 100-m depth-integrated Chl a increase (76–286 mg Chl a⋅m−2) from day −1 to day 21 (Figs. 1 A and B and 2B). The subsequent decline was due to mass mortality and sinking of some diatom species, which was partially compensated by continued accumulation of other species, reflected in the uninterrupted linear decline of Si* until the end of the experiment (Fig. 2A). Nitrate and DON concentrations declined by 1.6 and 1.8 mmol N⋅m−3, respectively, whereas the corresponding decline in Si was 11 (from 19 to 8) mmol Si⋅m−3 with an uptake ratio of Si/(DON + nitrate) of 3.2 and Si/nitrate of 6.9. This indicates that diatoms were responsible for new production and that the species involved were heavily silicified. Inside the patch, BSi/DC, BSi/POC, and BSi/PON ratios increased from 0.5 to 0.8, 0.2 to 0.5, and 1.3 to 2.7, respectively (Fig. 2 B and C). Similar ratios were found in iron-limited waters outside the patch (Fig. 2C). Assuming a diatom C/N ratio of 5, based on the slope of the linear regression between POC and PON inside the patch, the BSi/DN (diatom nitrogen) ratio increased from 2.5 to 4, implying that several of the dominant diatom species had Si/N ratios well above 4 to compensate for weaker silicification of other dominant species. The differential accumulation of BSi in the surface layer was due to a combination of sinking out of less-silicified diatoms and increasing populations of heavily silicified species. Our results support the finding that diatom community composition largely determines community silicification in the Southern Ocean (40). Although silica export did not increase in-patch compared with out-patch, iron-induced export of carbon with the sinking frustules amounted to 0.9 mol (10.8 g) C⋅m−2 during the flux event (35).
The populations of 45 of the 55 diatom taxa recorded during EIFEX increased their abundance inside the patch, indicating that artificial iron fertilization can stimulate growth of a broad range of diatom species. Maximum species-specific accumulation rates of the dominant species ranged between 0.03 and 0.10 d−1 (0.04–0.13 d−1 corrected for dilution) with highest rates associated with largest size and lowest mortality. The species-specific contribution to bloom biomass also depended on cell size, initial cell abundance (which varied by two orders of magnitude), and timing of the decline phase. As a result, the EIFEX bloom was highly diverse with 21 species contributing >85% of bloom biomass.
The species composition of nondiatom phytoplankton changed over the experiment, but, in contrast to the diatoms, their biomass remained more or less constant (Fig. 1B), apparently kept in check by grazing pressure of protozooplankton and copepods. The same trends and checks applied to the protozooplankton (Fig. S3), including specialized diatom grazers (e.g., Protoperidinium spp.), which are known to be preferred food of copepods (32). Tintinnid ciliates, despite protection by their loricae, were subject to heavier grazing pressure than diatoms as illustrated by the low ratio of full to empty intact and damaged loricae (Fig. 3D). The total consumption by microzooplankton (protozooplankton plus small copepods) estimated by the serial dilution method amounted to 17 g C⋅m−2 in 36 d, of which about 30% was provided by bacteria exploiting the DON pool (35). Bacterial biomass was stable but declined at the end of the experiment (Fig. 1B). The total bacterial carbon demand over the duration of EIFEX based on measured bacterial production and respiration amounted to 23 g⋅C m−2 of which about one-half was based on prefertilization DON (35), hence independent of primary production.
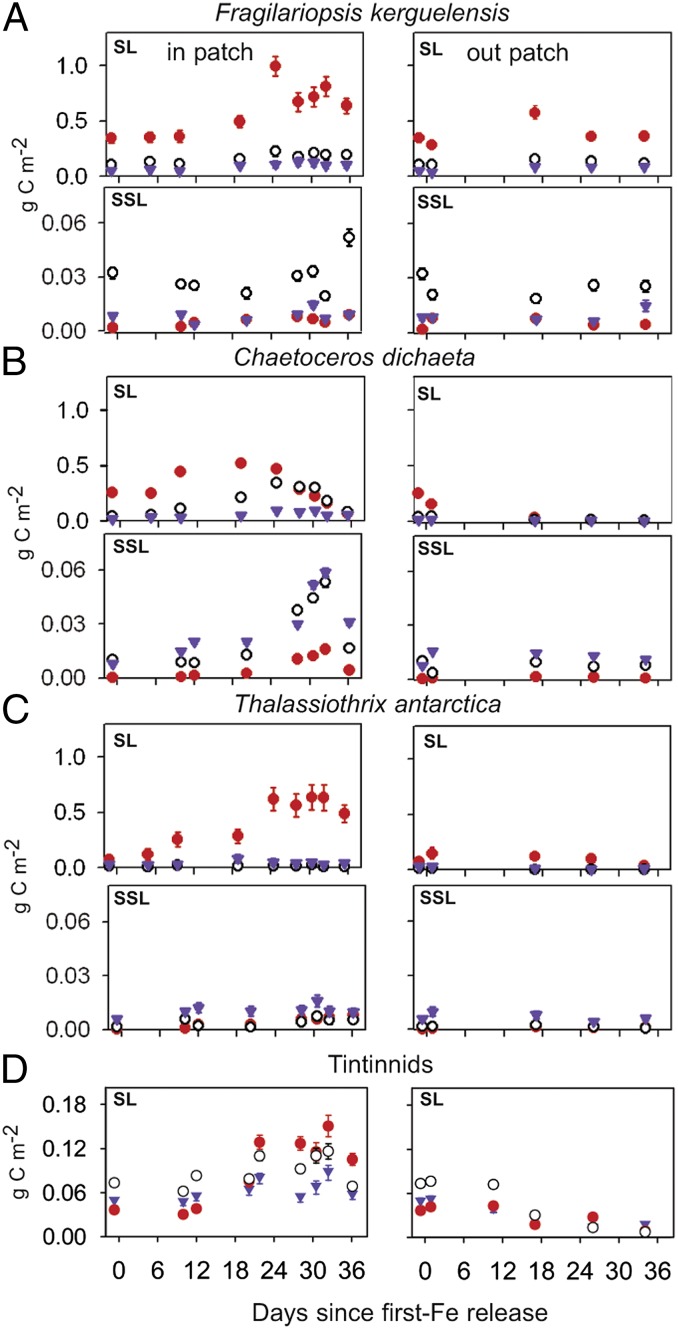
Fig. 3.
Stocks of three representative diatom species and total tintinnids in surface and subsurface layers. Integrated stocks of full (red filled circles), intact empty (black open circles), and broken diatom frustules and damaged tintinnid loricae (blue triangles) of (A) Fragilariopsis kerguelensis, (B) Chaetoceros dichaeta, (C) Thalassiothrix antarctica, and (D) total tintinnids. In each block, the left and right panels are integrated stocks inside and outside the patch, and upper and lower panels in the surface (SL: 0–100 m) and subsurface (SSL: 200–350 m) layers, respectively. Tintinnid stocks are shown for the SL only, and SSL values are in Fig. S2. Full frustules and loricae contain plasma. Empty and broken frustule and loricae numbers are presented as carbon equivalents for comparison with biomass in full frustules and living tintinnids, respectively; bars represent SEs. Note similarity between F. kerguelensis stocks inside and outside the patch in SL and SSL, in contrast to the differences in other species. This is supported by a one-sample one-sided t test applied to these data (Table S1).
Biomass of large (>1 mm) copepods increased until the end of the experiment, due to a combination of growth (development of larval stages) and upward migration from below 160 m. Total ingestion by copepods inside the fertilized patch over 36 d, estimated from fecal pellet production rates (Fig. S4A), amounted to 20 g C⋅m−2, of which 15 g C⋅m−2 was attributed to phytoplankton carbon indicated by gut evacuation experiments (Fig. S4B). Total primary production measured with the 14C-method amounted to 50 g C⋅m−2 over 36 d (35). Given the large uncertainties associated with all of the above rate measurements, the budget between primary production (50 g C⋅m−2), heterotrophic carbon consumption [∼12 g C⋅m−2 (microzooplankton grazing) + 15 g C⋅m−2 (copepod grazing) + ∼12 g C⋅m−2 (nitrate-based bacterial carbon demand) ∼ 40 g C⋅m−2], and carbon exported (10.8 g C⋅m−2) is reasonably well balanced. Although crushed and intact diatom frustules were prominent in pellets as well as in copepod guts (41), the relative grazing pressure on diatom populations estimated from ratios of full to empty and broken frustules was much lower than on other protists, exemplified here by tintinnid ciliates (Fig. 3D). Trends in 15N and 13C isotope ratios of suspended particulates indicated that the initial biomass increase was based primarily on nitrate uptake; following the flux event, recycled nitrogen contributed a larger fraction to community biomass (39).
Silica and Carbon Sinking Species.
The species comprising the diverse assemblage of large diatoms typical of the ACC differed in their impact on magnitude and composition of the vertical flux. This is illustrated by temporal trends in surface and subsurface layers of stocks and relative proportions of full, empty, and broken frustules of three representative species (Fig. 3). These different behavior patterns are of relevance to the silicon paradox. The surface-layer stocks of Fragilariopsis kerguelensis doubled inside the patch after 3 wk and maintained the new level for the next 2 wk. Stocks remained more or less constant outside the patch, whereas most other diatom species declined significantly. The contribution of F. kerguelensis to total diatom biomass outside the patch rose from 10% to 21% over the 5 wk, implying its superior survival ability under conditions of iron limitation but sufficient silicate and heavy copepod grazing pressure (Fig. 1C). In the subsurface layers below and outside the patch, stocks were fairly constant and at similar levels throughout (Fig. 3A), implying a steady, downward flux of largely empty chains and solitary frustules. This is consistent with the distribution of F. kerguelensis stocks in the deep-water column (Fig. 4A), which are threefold higher below 250 m than the deficit between peak and minimum stocks in the surface layer inside and outside the patch. The near constancy of the ratios of empty to broken frustules in the surface layers inside (2.1 ± 0.4) and outside the patch (2.0 ± 0.5), also recorded in a previous experiment (EisenEx) conducted in spring (36), provides further support for controlled, low-level, quasiconstant mortality in this species with sinking out of empty frustules and recycling of cytoplasm in the surface layer (Fig. S5).
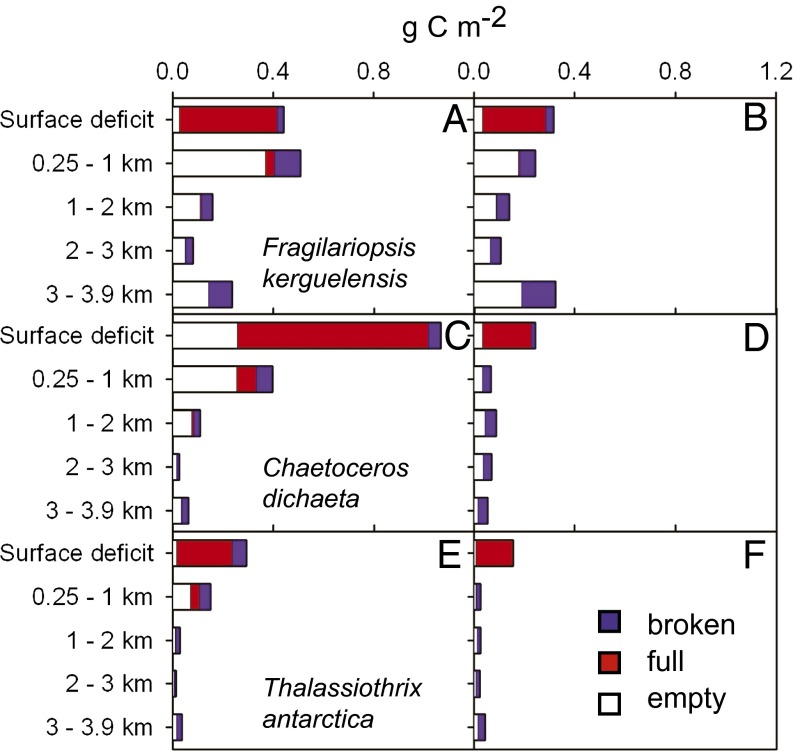
Fig. 4.
Stocks of three representative diatom species in deep layers in comparison with the surface deficit. Inventories of full (red bars), intact empty (white bars), and broken (blue bars) frustules of (A and B) Fragilariopsis kerguelensis, (C and D) Chaetoceros dichaeta, and (E and F) Thalassiothrix antarctica. The surface deficit was estimated as the difference between peak stocks and the stocks present at the station of the deep profile. Left panels, inside the patch; right panels, outside the patch. The deep in-patch and out-patch profiles were taken on days 36 and 34, respectively, 6–8 d after the flux event commenced. The higher values of A and B in the deepest layer are partly due to resuspension of sediment in the nepheloid layer. The unexpected low biomass of C. dichaeta in deep layers under the patch is due to undersampling by Niskin bottles of large, rapidly sinking aggregates in which they were packed.
The boom-and-bust behavior of a group of disparate species exemplified by Chaetoceros dichaeta (Fig. 3B) contrasted with the persistent strategy of Fragilariopsis kerguelensis. The former species underwent mass mortality in the surface layer, signaled by a sudden increase in empty frustules, followed by a fivefold population decrease due to sinking. The flux event triggered by mass mortality of C. dichaeta transported ∼10.8 g C⋅m−2 as rapidly sinking aggregates (Fig. S6 A–C) into the deep-water column during the last 10 d (35). Sticky, autolyzed cytoplasm, stretched into mucoid sheets along the spines of various species, entangled chains with one another into millimeter-sized aggregates (Fig. S6 A and B). The process was accompanied by increased levels of transparent exopolymer particles (Fig. S7) in ambient water. Chains of various species were entrained in the aggregates (Fig. S6 B and C) and mass presence of Chaetoceros spp. was only observed under the patch during the flux event, whereas they were at low levels outside it (Figs. 3B and 4 C and D and Fig. S6 D and E). However, frustule stocks in the deep-water column under the patch were lower than the surface deficit (unlike F. kerguelensis) and the bulk was found in the upper 1,000 m (Fig. 4C). The depth distribution inferred here from discrete 12-L water samples differed from transmissometer profiles, which recorded a rapid increase in particle stocks in the entire deep-water column during the flux event, albeit with fairly uniform depth distribution (Fig. S8). The discrepancy can be attributed to undersampling by Niskin bottles of large, rapidly sinking aggregates that constituted the bulk of flux below 1,000 m.
The common feature of the remaining, heterogeneous group of largely centric colonial species (in particular Corethron inerme and Proboscia alata), but exemplified here by the heavily silicified pennate Thalassiothrix antarctica (Fig. 3C), was the low mortality rate (the ratio of full to empty and broken frustules increased from initially 1.9 to 17.6 by the end of the experiment), which resulted in (i) stable or growing populations until the end of the experiment, (ii) the highest accumulation rates during the growth phase, and (iii) low concentrations in the subsurface and deep layers (Figs. 3C and 4 E and F). The low sinking losses indicate that living cells maintain neutral buoyancy despite massive ballast in the thick cell walls of some species. Because grazing pressure on these species was low, it is likely that a substantial portion of their biomass will have eventually sunk out, indicated by satellite images of fading Chl a concentrations in the patch after day 36 (35). Mass sinking events of T. antarctica have been reported from the ACC and, presumably because of its thick cell walls, it is a major constituent of AZ sediments (16, 17).
The behavior of Fragilariopsis kerguelensis and Chaetoceros dichaeta reflect two distinctly different life cycle strategies characteristic of the pelagic realm: (i) maintenance of relatively constant stocks in the surface layer (persistence strategy) and (ii) cycles of biomass buildup during favorable growth conditions, followed by mass mortality and rapid population decline (boom-and-bust strategy). These categories represent end points of a gradient along which other ACC diatoms fall. Whereas species of the persistent strategy generally occur at background levels (42), F. kerguelensis is a dominant species, which increases in abundance, together with copepod biomass, northward across the AZ (14, 16, 27, 36, 37). This distribution can be explained by its ability to withstand grazing pressure apparently due to its extraordinarily strong frustules (43), which reduces the proportion of cells cracked and crushed by copepod feeding (Fig. S5 B and C) and facilitates viable gut passage (41, 44). Its increasing abundance across the AZ is concomitant with declining Si* and increasing deposition of its frustules in underlying sediments (Fig. S5D; see also refs. 16, 25, and 45). In summary, this species, together with a few others (see below), is a silica sinker responsible for selective silicon export and burial coupled with carbon and nitrogen retention across the surface layer of the iron-limited AZ. Coevolution between diatoms and copepods, which selects for traits at the upper limit of the handling capacity of the herbivores, hence could explain the heavy silicification resulting in high Si/C ratios of open-ocean ACC diatoms (14).
In contrast, species with a boom-and-bust strategy that undergo mass mortality and produce aggregates in the surface layer are carbon sinkers (14, 27, 46) with spiny and needle-shaped species, in particular the genus Chaetoceros, most likely to form the large, rapidly sinking aggregates that are responsible for the deepest flux (47). Layers of diatom fluff, widely reported on the seafloor in the aftermath of surface blooms, will be caused by such flux events (48). In the ACC, blooms of boom-and-bust species are local, and characteristic of iron-sufficient waters in the proximity of land masses and in regions of dust deposition (49–51).
Zonally persistent species like Fragilariopsis kerguelensis are apparently geared to the copepod-dominated recycling system, which also recycles iron (52). It follows that high sedimentary silica accumulation rates are a proxy for iron-limited diatom assemblages rather than for high surface productivity, contrary to the prevailing view (33, 53). As the bulk of the frustules of carbon-sinking species are not buried (e.g., Pseudo-nitzschia and vegetative cells of Chaetoceros and Thalassiosira except T. lentiginosa), they fuel the water column silicon trap, which is partially depleted by burial of exceptionally robust frustules of F. kerguelensis and a few other species such as Thalassiosira lentiginosa, Thalassiothrix antarctica, and Thalassionema nitzschioides (16, 25, 45).
During the Last Glacial Maximum (LGM), natural iron fertilization extended the current range of carbon-sinking species from the Antarctic Peninsula across the entire South Atlantic sector of the ACC (54). It is likely that artificial iron fertilization will have a similar effect, i.e., it will extend populations of carbon-sinking species over a larger area that will sink more carbon per silicon than is currently the case. Nevertheless, the upper limit of carbon sequestration will be determined by how much of the upwelling silicate is taken up and exported by carbon-sinking species before exhaustion by silica-sinking species. Given their persistent life cycle strategy geared to the surface recycling system and the silicon-sequestering circulation pattern of the ACC, silica sinkers will always be present in the ACC. Thus, the sedimentary band dominated by Fragilariopsis kerguelensis moved north in the LGM (54), implying that its northern boundary will have coincided with the zone of summer surface silicate limitation as is the case today along the APF (18). Indeed, we suggest that such a vertical silicon retention loop, maintained by diatoms and copepods strikingly similar to those of the AZ (14), could also be responsible for silicon trapping and burial in the HNLC region of the subarctic North Pacific (4).
Materials and Methods
Water samples were taken with 12-L Niskin bottles mounted on a CTD rosette equipped with a profiling transmissometer and treated according to standard protocols for the various measurements. Prokaryotic abundance was determined by enumerating DAPI-stained cells under the epifluorescence microscope. All eukaryotic organisms were identified as far as possible to the species level and counted by light microscopy. Larger protozoa and copepods <1 mm (including larvae) were concentrated by passing 12 or 24 L through 20-µm mesh gauze. Diatoms and other suspended particles in the deep-water column below 200 m were concentrated over 10-µm mesh gauze. Zooplankton >1 mm were sampled with vertical net tows taken between 0- and 160-m depth to include vertically migrating populations. All organism counts were converted into carbon units using standard protocols. Detailed methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are indebted to the captain and crew of RV Polarstern. We thank N. Cassar, J. E. Cloern, D. Iudicone, M. G. Mazzocchi, and M. Ribera d’Alcalá for comments on the manuscript. We are thankful to Uta Passow for her support with the transparent exopolymer particle analysis. P.A. was supported through Deutsche Forschungsgemeinschaft–Cluster of Excellence “The Ocean in the Earth System” and the Centre for Ice, Climate and Ecosystems at the Norwegian Polar Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data have been deposited in the PANGAEA database, www.pangaea.de/.
See Commentary on page 20358.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309345110/-/DCSupplemental.
References
- 1.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281(5374):200–207. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 2.Smetacek V. Diatoms and the ocean carbon cycle. Protist. 1999;150(1):25–32. doi: 10.1016/S1434-4610(99)70006-4. [DOI] [PubMed] [Google Scholar]
- 3.Dugdale RC, Wilkerson FP. Sources and fates of silicon in the ocean: The role of diatoms in the climate and glacial cycles. Sci Mar. 2001;65(Suppl 2):141–152. [Google Scholar]
- 4.Sarmiento JL, Gruber N, Brzezinski MA, Dunne JP. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature. 2004;427(6969):56–60. doi: 10.1038/nature02127. [DOI] [PubMed] [Google Scholar]
- 5.Pollard R, Treguer P, Read J. Quantifying nutrient supply to the Southern Ocean. J Geophys Res. 2006;111(C5):C05011. [Google Scholar]
- 6.Coale KH, et al. Southern Ocean iron enrichment experiment: Carbon cycling in high- and low-Si waters. Science. 2004;304(5669):408–414. doi: 10.1126/science.1089778. [DOI] [PubMed] [Google Scholar]
- 7.Brzezinski MA. The Si-N-C ratio of marine diatoms. Interspecific variability and the effect of some environmental variables. J Phycol. 1985;21(3):347–357. [Google Scholar]
- 8.Sarthou G, Timmermans KR, Blain S, Treguer P. Growth physiology and fate of diatoms in the ocean: A review. J Sea Res. 2005;53(1-2):25–42. [Google Scholar]
- 9.Pondaven P, et al. Resolving the “opal paradox” in the Southern Ocean. Nature. 2000;405(6783):168–172. doi: 10.1038/35012046. [DOI] [PubMed] [Google Scholar]
- 10.Takeda S. Influence of iron availability on nutrient consumption ratio of diatoms in oceanic waters. Nature. 1998;393(6687):774–777. [Google Scholar]
- 11.Hutchins DA, Bruland KW. Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature. 1998;393(6685):561–564. [Google Scholar]
- 12.Franck VM, Bruland KW, Hutchins DA, Brzezinski MA. Iron and zinc effects on silicic acid and nitrate uptake kinetics in three high-nutrient, low-chlorophyll (HNLC) regions. Mar Ecol Prog Ser. 2003;252:15–33. [Google Scholar]
- 13.Marchetti A, Cassar N. Diatom elemental and morphological changes in response to iron limitation: A brief review with potential paleoceanographic applications. Geobiology. 2009;7(4):419–431. doi: 10.1111/j.1472-4669.2009.00207.x. [DOI] [PubMed] [Google Scholar]
- 14.Smetacek V, Assmy P, Henjes J. The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles. Antarct Sci. 2004;16(4):541–558. [Google Scholar]
- 15.Hoffmann LJ, Peeken I, Lochte K. Effects of iron on the elemental stoichiometry during EIFEX and in the diatoms Fragilariopsis kerguelensis and Chaetoceros dichaeta. Biogeosciences. 2007;4(4):569–579. [Google Scholar]
- 16.Zielinski U, Gersonde R. Diatom distribution in Southern Ocean surface sediments (Atlantic sector): Implications for paleoenvironmental reconstructions. Palaeogeogr Palaeoclimatol Palaeoecol. 1997;129(3-4):213–250. [Google Scholar]
- 17.Kemp AES, et al. Production of giant marine diatoms and their export at oceanic frontal zones: Implications for Si and C flux from stratified oceans. Global Biogeochem Cycles. 2006;20(4):GB4S04. [Google Scholar]
- 18.Cortese G, Gersonde R. Plio/Pleistocene changes in the main biogenic silica carrier in the Southern Ocean, Atlantic Sector. Mar Geol. 2008;252(3-4):100–110. [Google Scholar]
- 19.Tréguer P, et al. The silica balance in the world ocean: A reestimate. Science. 1995;268(5209):375–379. doi: 10.1126/science.268.5209.375. [DOI] [PubMed] [Google Scholar]
- 20.DeMaster DJ. The accumulation and cycling of biogenic silica in the Southern Ocean: Revisiting the marine silica budget. Deep Sea Res Part II Top Stud Oceanogr. 2002;49(16):3155–3167. [Google Scholar]
- 21.Blain S, et al. Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature. 2007;446(7139):1070–1074. doi: 10.1038/nature05700. [DOI] [PubMed] [Google Scholar]
- 22.Pollard RT, et al. Southern Ocean deep-water carbon export enhanced by natural iron fertilization. Nature. 2009;457(7229):577–580. doi: 10.1038/nature07716. [DOI] [PubMed] [Google Scholar]
- 23.Cassar N, et al. The Southern Ocean biological response to aeolian iron deposition. Science. 2007;317(5841):1067–1070. doi: 10.1126/science.1144602. [DOI] [PubMed] [Google Scholar]
- 24.Seiter K, Hensen C, Schroter E, Zabel M. Organic carbon content in surface sediments—defining regional provinces. Deep Sea Res Part I Oceanogr Res Pap. 2004;51(12):2001–2026. [Google Scholar]
- 25.Crosta X, Pichon J-J, Labracherie M. Distribution of Chaetoceros resting spores in modern peri-Antarctic sediments. Mar Micropaleontol. 1997;29(3-4):283–299. [Google Scholar]
- 26.Armand LK, Crosta X, Quéguiner B, Mosseri J, Garcia N. Diatoms preserved in surface sediments of the northeastern Kerguelen Plateau. Deep Sea Res Part II Top Stud Oceanogr. 2008;55(5-7):677–692. [Google Scholar]
- 27.Mosseri J, Quéguiner B, Armand L, Cornet-Barthaux V. Impact of iron on silicon utilization by diatoms in the Southern Ocean: A case study of Si/N cycle decoupling in a naturally iron-enriched area. Deep Sea Res Part II Top Stud Oceanogr. 2008;55(5-7):801–819. [Google Scholar]
- 28.Parsons TR, Lalli CM. Comparative oceanic ecology of the plankton communities of the subarctic Atlantic and Pacific oceans. Oceanogr Mar Biol Annu Rev. 1988;26:317–359. [Google Scholar]
- 29.Pollard RT, Bathmann U, Dubischar C, Read JF, Lucas M. Zooplankton distribution and behaviour in the Southern Ocean from surveys with a towed Optical Plankton Counter. Deep Sea Res Part II Top Stud Oceanogr. 2002;49(18):3889–3915. [Google Scholar]
- 30.Frost BW. The role of grazing in the nutrient-rich areas of the open sea. Limnol Oceanogr. 1991;36(8):1616–1630. [Google Scholar]
- 31.Turner JT. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol. 2002;27(1):57–102. [Google Scholar]
- 32.Irigoien X, Flynn KJ, Harris RP. Phytoplankton blooms: A “loophole” in microzooplankton grazing impact? J Plankton Res. 2005;27(2):313–321. [Google Scholar]
- 33.Sigman DM, Hain MP, Haug GH. The polar ocean and glacial cycles in atmospheric CO2 concentration. Nature. 2010;466(7302):47–55. doi: 10.1038/nature09149. [DOI] [PubMed] [Google Scholar]
- 34.Smetacek V, Naqvi SWA. The next generation of iron fertilization experiments in the Southern Ocean. Philos Trans A Math Phys Eng Sci. 2008;366(1882):3947–3967. doi: 10.1098/rsta.2008.0144. [DOI] [PubMed] [Google Scholar]
- 35.Smetacek V, et al. Deep carbon export from a Southern Ocean iron-fertilized diatom bloom. Nature. 2012;487(7407):313–319. doi: 10.1038/nature11229. [DOI] [PubMed] [Google Scholar]
- 36.Assmy P, Henjes J, Klaas C, Smetacek V. Mechanisms determining species dominance in a phytoplankton bloom induced by the iron fertilization experiment EisenEx in the Southern Ocean. Deep Sea Res Part I Oceanogr Res Pap. 2007;54(3):340–362. [Google Scholar]
- 37.Bathmann UV, Scharek R, Klaas C, Dubischar CD, Smetacek V. Spring development of phytoplankton biomass and composition in major water masses of the Atlantic sector of the Southern Ocean. Deep Sea Res Part II Top Stud Oceanogr. 1997;44(1-2):51–67. [Google Scholar]
- 38.von Harbou L. 2009. Trophodynamics of salps in the Atlantic Southern Ocean. PhD thesis (Univ of Bremen, Bremen, Germany). Available at http://nbn-resolving.de/urn:nbn:de:gbv:46-diss000119205. Accessed October 2, 2013.
- 39.Berg GM, et al. Variation in particulate C and N isotope composition following iron fertilization in two successive phytoplankton communities in the Southern Ocean. Global Biogeochem Cycles. 2011;25:Gb3013. [Google Scholar]
- 40.Baines SB, Twining BS, Brzezinski MA, Nelson DM, Fisher NS. Causes and biogeochemical implications of regional differences in silicification of marine diatoms. Glob Biogeochem Cycles. 2010;24:GB4031. [Google Scholar]
- 41.Kruse S, Jansen S, Krägefsky S, Bathmann U. Gut content analyses of three dominant Antarctic copepod species during an induced phytoplankton bloom EIFEX (European iron fertilization experiment) Mar Ecol (Berl) 2009;30(3):301–312. [Google Scholar]
- 42.Yooseph S, et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature. 2010;468(7320):60–66. doi: 10.1038/nature09530. [DOI] [PubMed] [Google Scholar]
- 43.Hamm CE, et al. Architecture and material properties of diatom shells provide effective mechanical protection. Nature. 2003;421(6925):841–843. doi: 10.1038/nature01416. [DOI] [PubMed] [Google Scholar]
- 44.Hamm C, Smetacek V. Armor: Why, when and how. In: Falkowski PG, Knoll AH, editors. Evolution of Primary Producers in the Sea. Burlington, MA: Elsevier Academic; 2007. pp. 311–332. [Google Scholar]
- 45.Esper O, Gersonde R, Kadagies N. Diatom distribution in southeastern Pacific surface sediments and their relationship to modern environmental variables. Palaeogeogr Palaeocl. 2010;287(1-4):1–27. [Google Scholar]
- 46.Green SE, Sambrotto RN. Plankton community structure and export of C, N, P and Si in the Antarctic Circumpolar Current. Deep Sea Res Part II Top Stud Oceanogr. 2006;53(5-7):620–643. [Google Scholar]
- 47.Riebesell U, Wolf-Gladrow DA. The relationship between physical aggregation of phytoplankton and particle flux: A numerical model. Deep Sea Res. 1992;39(7-8):1085–1102. [Google Scholar]
- 48.Beaulieu SE. Accumulation and fate of phytodetritus on the sea floor. In: Gibson RN, Barnes M, Atkinson RJ, editors. Oceanography and Marine Biology: An Annual Review. London: Taylor & Francis; 2002. pp. 171–232. [Google Scholar]
- 49.Armand LK, Cornet-Barthaux V, Mosseri J, Quéguiner B. Late summer diatom biomass and community structure on and around the naturally iron-fertilised Kerguelen Plateau in the Southern Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2008;55(5-7):653–676. [Google Scholar]
- 50.Poulton AJ, et al. Phytoplankton community composition around the Crozet Plateau, with emphasis on diatoms and Phaeocystis. Deep Sea Res Part II Top Stud Oceanogr. 2007;54(18-20):2085–2105. [Google Scholar]
- 51.Salter I, et al. Diatom resting spore ecology drives enhanced carbon export from a naturally iron-fertilized bloom in the Southern Ocean. Global Biogeochem Cycles. 2012;26 GB1014. [Google Scholar]
- 52.Boyd PW, Ellwood MJ. The biogeochemical cycle of iron in the ocean. Nat Geosci. 2010;3(10):675–682. [Google Scholar]
- 53.Anderson RF, et al. Wind-driven upwelling in the Southern Ocean and the deglacial rise in atmospheric CO2. Science. 2009;323(5920):1443–1448. doi: 10.1126/science.1167441. [DOI] [PubMed] [Google Scholar]
- 54.Abelmann A, Gersonde R, Cortese G, Kuhn G, Smetacek V. Extensive phytoplankton blooms in the Atlantic sector of the glacial Southern Ocean. Paleoceanography. 2006;21(1):PA1013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.