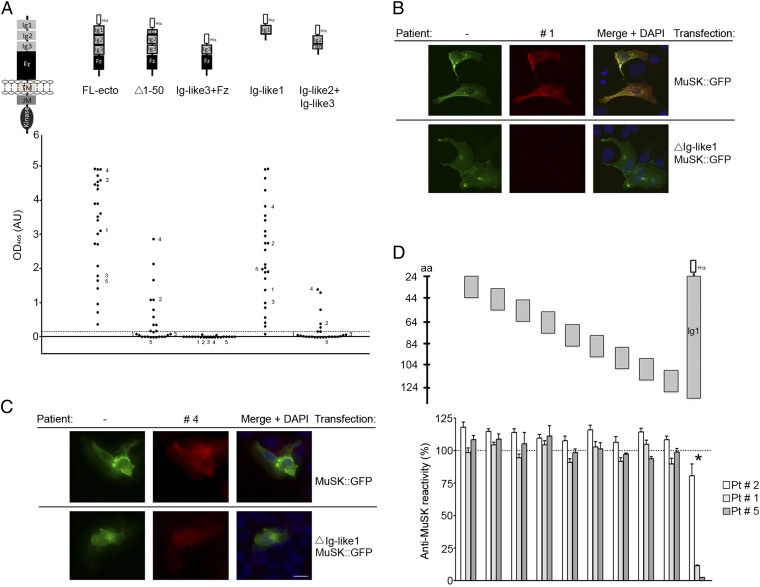
Fig. 1.
MuSK MG IgG4 antibodies bind predominantly to the first Ig-like domain in MuSK. (A) An ELISA shows that antibodies from all 25 patients bind to the extracellular region of MuSK. The predominant binding sites reside in the first Ig-like domain, because antibodies bind to this domain nearly as well as the entire extracellular region. Moreover, deletion of the N-terminal half of the first Ig-like domain substantially reduces antibody binding. Antibodies from five patients have additional reactivity to the second Ig-like domain. Data shown reflect the average binding per patient determined in three independent assays (Fig. S1). (B and C) Antibodies that bind selectively to the first Ig-like domain stain cells expressing full-length MuSK-GFP but not ∆Ig-like1-MuSK-GFP, whereas antibodies with additional reactivity bind to cells expressing either construct. (D) An ELISA shows that antibody-binding to the extracellular region of MuSK is strongly inhibited by the first Ig-like domain but poorly by 20-mer overlapping peptides from this domain.