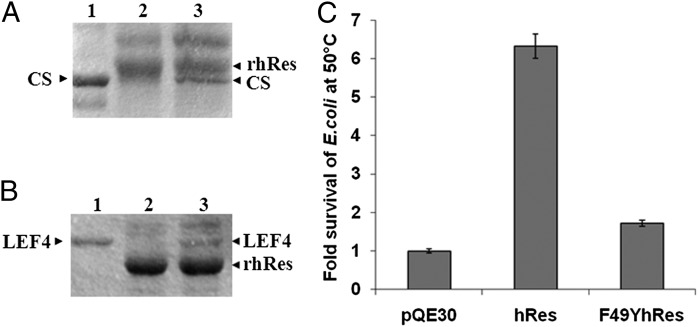
Fig. 3.
rhRes binds only to nonnative proteins, and hRes can rescue E. coli cells from thermal shock. (A) Coimmunoprecipitation of rhRes with native and denatured CS. The different lanes are the following: native CS (lane 1); coimmunoprecipitation of rhRes after incubating with native CS (lane 2); or with GdnHCl-denatured CS (lane 3). (B) Coimmunoprecipitation of rhRes with native and denatured LEF4. Native LEF4 (lane 1); coimmunoprecipitation of rhRes after incubating with native LEF4 (lane 2); or with GdnHCl-denatured LEF4 (lane 3). Arrowheads indicate relative position of the proteins. The gel pictures are representative of three independent experiments. (C) E. coli M15 cells were transformed with plasmid pQE30, pQE30hRes, or pQE30F49YhRes. Note that after heat treatment for 45 min at 50 °C, E. coli M15 cells transformed with hRes showed a more than sevenfold survival compared with pQE30 vector control, whereas those transformed with mutant F49YhRes showed significantly reduced survival compared with wild-type hRes. Each experiment was carried out in triplicate. The error bars represent SEM.