Significance
Societies value most natural ecosystems because they provide many different individual benefits all at once. However, species extinctions have threatened ecosystems' ability to function this way. Our study provides a real-world example that the loss of key species from a salt marsh can have profound impacts on the overall performance of an ecosystem. Our findings also suggest that the ability for nature to perform well at multiple levels may depend on having many distantly related species present, increasing the chances of maximizing different functions and services. Salt marshes provide many services to humans, including water filtration and coastal buffering, and this study shows that these processes are likely enhanced by the presence of a diverse community of consumers.
Keywords: multifunctionality, fungus, Sesarma reticulatum, Litoraria irrorata
Abstract
The global biodiversity crisis impairs the valuable benefits ecosystems provide humans. These nature-generated benefits are defined by a multitude of different ecosystem functions that operate simultaneously. Although several studies have simulated species loss in communities and tracked the response of single functions such as productivity or nutrient cycling, these studies have involved relatively similar taxa, and seldom are strikingly different functions examined. With the exception of highly managed ecosystems such as agricultural fields, rarely are we interested in only one function being performed well. Instead, we rely on ecosystems to deliver several different functions at the same time. Here, we experimentally investigated the extinction impacts of dominant consumers in a salt marsh. These consumers are remarkably phylogenetically diverse, spanning two kingdoms (i.e., Animalia and Fungi). Our field studies reveal that a diverse consumer assemblage significantly enhances simultaneous functioning of disparate ecosystem processes (i.e., productivity, decomposition, and infiltration). Extreme functional and phylogenetic differences among consumers underlie this relationship. Each marsh consumer affected at least one different ecosystem function, and each individual function was affected by no more than two consumers. The implications of these findings are profound: If we want ecosystems to perform many different functions well, it is not just number of species that matter. Rather, the presence of species representing markedly different ecologies and biology is also essential to maximizing multiple functions. Moreover, this work emphasizes the need to incorporate both microcomponents and macrocomponents of food webs to accurately predict biodiversity declines on integrated-ecosystem functioning.
Biodiversity loss decreases the efficiency of ecosystem functions and reduces the magnitude and quality of services natural ecosystems provide (1–8). When measuring functions individually, experimental investigations indicate that having a diverse species assemblage can maximize certain processes (2). Despite these significant results, focusing on single functions is an unrealistic estimate of the effects of the global biodiversity crisis because most natural habitats are valued for performing many functions and services (9). The few studies that have investigated how species loss affects the simultaneous performance of multiple functions (i.e., multifunctionality) suggest that many species are required to maintain high levels of multifunctionality (5, 10–14). These studies, however, are often highly artificial relative to the real world. Study designs include only plants or microbes, occur in microcosms or mesocosms, or measure highly related functions. Thus, the hypothesis that more diverse species assemblages lead to increased function of disparate processes remains experimentally untested in a natural setting that simulates changes in the dominant species most vulnerable to extinction (i.e., consumers; ref. 15).
Consumers, such as grazers and apex predators, play important roles in most natural communities (e.g., kelps, seagrasses, coral reefs, tropical and temperate forests, grasslands, salt marshes, rocky shores, oyster reefs), regulating both key processes and habitat structure (16–20). From many studies, we know that differences in consumer composition (i.e., species richness, species identity) can affect performance of single ecosystem functions, including primary and secondary production, decomposition, and consumption rate across trophic levels (2). Although some studies have taken important steps to better reflect real-world scenarios (21) (e.g., studying species extinctions rather than additions) (22), all studies have been limited in taxonomic scope, focusing on either macro- or microbial organisms. Because micro and macro consumers can regulate a wide range of ecosystem functions and commonly interact in nature (e.g., through facilitation, competition, parasitism), investigating the individual and interactive effects of phylogenetically variable assemblages of dominant consumers on ecosystem function is key to understanding realistic impacts of the continued increase in species extinctions (23, 24).
In this study, we examine the relationship between consumer variety and overall ecosystem functioning in a Southeastern US salt marsh dominated by the cordgrass Spartina alterniflora (hereafter Spartina). In an 8-mo field experiment, we manipulated the occurrences of the three most abundant salt marsh consumers: the snail Litoraria irrorata, the crab Sesarma reticulatum, and fungi (including Mycosphaerella sp. and Phaeospheria spartinicola) that can consume both live and dead plant material (25, 26), and measured effects on three separate ecosystem functions for which coastal wetlands are highly valued. To assess the importance of an intact consumer assemblage in maintaining marsh ecosystem functions simultaneously, we quantified the average rate of decomposition, net primary production, and infiltration (13, 14, 27) and assessed whether these functions exceeded a performance threshold (11, 12) across different combinations of salt marsh consumers.
Results
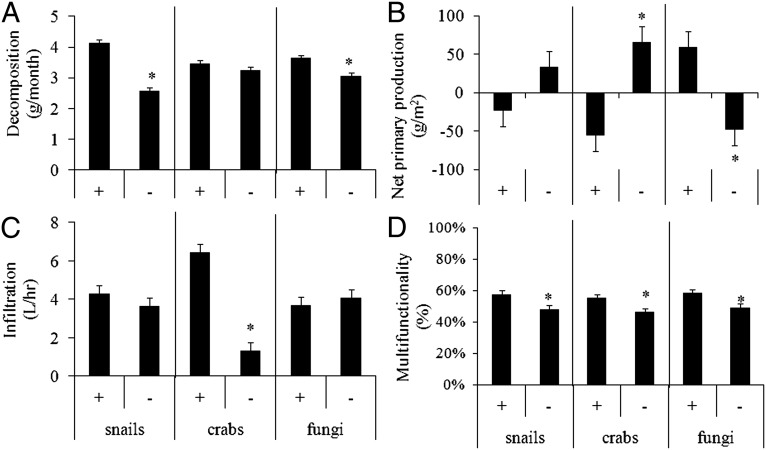
We assessed the effect of each consumer on ecosystem functioning by quantifying the main effect of each species (crab, snail, fungi) because we found no statistical interactions between consumers. Decomposition rate, measured in grams of dead Spartina removed per month, was affected by removal of snails and fungicide application (Fig. 1A). Decomposition rate was 1.6× slower when snails were removed (P < 0.0001) and 1.2× slower when fungicide was added (P < 0.0001). Net primary production (NPP) was most affected by crabs and fungicide (Fig. 1B); crab removal increased production by 207% (P < 0.0001) and fungicide application decreased production by 120% (P = 0.0004). Snail removal did not have a significant effect on NPP (P = 0.051), but there was a trend of increased plant growth. Crab removal was the only treatment that affected marsh infiltration, reducing infiltration rate by 80% (Fig. 1C; P < 0.0001), which has been shown to negatively affect overall marsh biogeochemistry and slow nutrient transfer (28).
Fig. 1.
Effect of each consumer removal (-) and consumer presence (+). on decomposition rate (A), NPP (B), infiltration (C), and average multifunctionality (D). Means and SEM confidence intervals are from pair-wise comparison of main effects of consumer removals from three-way ANOVAs (crab × snail × fungicide), and asterisk represents significant (P < 0.0001) differences from post hoc Tukey tests between removal (-) and present (+) for each consumer.
Multifunctionality, measured as the geometric mean of all three functions, was affected by all consumers (Fig. 1D). Snail removal decreased multifunctionality by 16% (P = 0.0017), crab removal decreased multifunctionality by 16% (P < 0.0001), and fungicide application decreased multifunctionality by 15% (P < 0.0001), revealing that both macroconsumers and microconsumers played important roles in enhancing ecosystem multifunctionality.
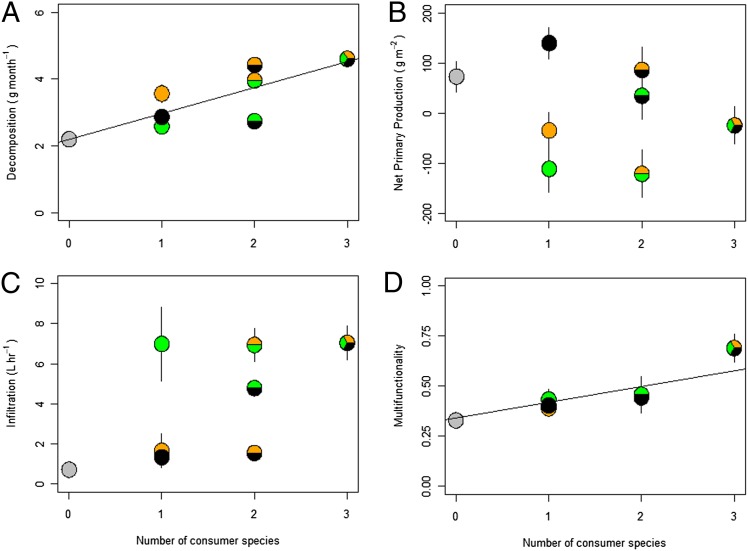
To determine how species richness affected ecosystem functioning, irrespective of the identity of species included in treatments of a given richness level, we grouped treatments into four levels (i.e., zero, one, two, or three consumers, n = 8, 24, 24, 8 respectively) and performed regressions with each single function and average multifunctionality (Fig. 2 and Figs. S1 and S2). Additionally, we calculated Dmax, an overyielding criterion that evaluates whether single species (Dmax ≤ 0) or three species treatments, irrespective of species composition (Dmax ≥ 0), drive a significant effect of number of consumer species on each function (Table 1) (29). Decomposition was the only ecosystem function significantly affected by species number, where each addition of one consumer resulted in a 14% rise in decomposition rate (P < 0.0001; Fig. 2A and Fig. S1A). Decomposition was strongly influenced by overyielding (Table 1), because fungi and snails had particularly strong, positive effects on decomposition rate (Fig. 1A). NPP was not significantly affected by number of consumers (P = 0.2344; Fig. 2B and Fig. S1B), likely because the negative effect of fungicide application was cancelled out by the positive effect of crab removal in two and three species treatments (Fig. 1A). Infiltration was only increased by crab treatments (Fig. 1C) and was not affected by species richness (P = 0.0604; Fig. 2C and Fig. S1C).
Fig. 2.
Mean effect of each treatment per number of consumer species present on ecosystem function. A floating pie representation of each treatment reveals which one and two species treatments were responsible for the trends found in Fig. S1. Floating circles represent the mean value of each of the eight treatments (n = 8 for A and B, n = 4 for C and D) for all response variables from our field experiment. Orange circles represent snail-only treatments, green circles represent crab-only treatments, and black circles represent fungus-only treatments. Two species treatments are represented as a combinantion of the colors from one species treatments (e.g., the green/black circle represents crab and fungus together treatment). Increasing number of consumer species had variable effects on regressions of single ecosystem functions decomposition (A), net primary production (B), and infiltration (C), and had a significant positive effect on multifunctionality (D).
Table 1.
Coefficient table from regression of number of consumer species and ecosystem functions
| Function | m | SE | t | P | R2 | Dmax |
| Decomposition, g/mo | 0.87 | 0.19 | 4.67 | <0.0001 | 0.26 | 0.292 |
| NPP, g/m2 | −19.7 | 20.11 | −0.98 | 0.3305 | 0.0153 | −0.364 |
| Infiltration, L/h | 1.15 | 0.59 | 1.95 | 0.0604 | 0.113 | 0.008 |
| Multifunctionality, % | 0.10 | 0.02 | 4.11 | 0.0003 | 0.534 | 0.270 |
Significant P and R2 values (at an α level of 0.05) are bolded. Dmax > 0 indicates that overyielding contributes to the effect of diversity on functioning.
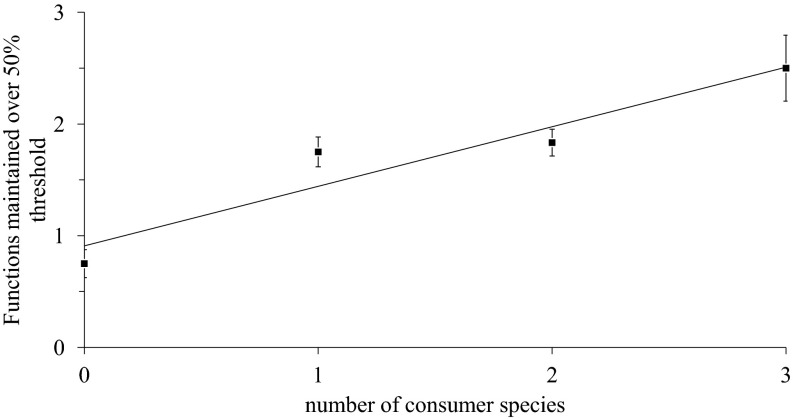
Although the effect of consumer removals on single functions varied in sign and strength, the intact consumer assemblage (i.e., crab, snail, and fungus present) strongly enhanced the average rate of all marsh functions simultaneously (i.e., average multifunctionality), where every consumer added increased functioning by 11% (Fig. 2D, Table 1, and Fig. S1D; P = 0.0003). Multifunctionality measurements also exhibited the smallest amount of variance among all measures of ecosystem function (R2 = 0.534). We also assessed the ability for treatments to achieve a multifunctionality threshold that, like a prior study (30), we defined as a value that is 50% of each function’s observed maximum value. Number of consumer species had a significant, positive effect on the number of functions maintained above the multifunctionality threshold (P < 0.0001, R2 = 0.2370; Fig. 3). The inclusion of each additional consumer species yielded, on average, an increase in 0.4 ± 0.09 functions maintained over the 50% threshold (t = 4.39, P < 0.0001). In fact, only intact assemblages were able to maintain all functions over the threshold, and the probability of a given plot maintaining the threshold decreased as species loss and number of functions considered increased (Fig. S2; P < 0.0001).
Fig. 3.
Multifunctionality threshold. The number of functions maintained over 50% threshold increases as the number of salt marsh consumer species increases. Values and SEM confidence intervals are the mean number of functions maintained over 50% threshold per level of diversity.
Discussion
Here, through the manipulation of a wide phylogenetic range of consumers, we experimentally demonstrate that when species known to play a major role in a natural community are lost, the consequences of cumulative losses depends on whether more than one ecosystem function is examined. Further, if those species are both commonly dominant and functionally distinct, each individual loss will have a profound effect because each loss can decrease the rate of a different function. In fact, the functional diversity of the salt marsh consumer assemblage was likely driven by the cross-kingdom variety of its consumers, because: (i) the removal of the microbial consumer or either animal consumer had nearly equal effects on our metrics of overall ecosystem functioning and (ii) the unique functional diversity elicited by each consumer was closely linked to the defining natural history of their higher order taxonomy. Because human society places value on a great number of diverse services (e.g., fisheries production, runoff filtration, coastal protection) generated by coastal ecosystems, we value these ecosystems not by the performance of just one or related functions, but rather we seek natural ecosystems that perform very different functions and services well at the same time (9). If we are to manage ecosystems and understand the realistic effect of species loss in the field, our results suggest we should pay particular attention to both the functional diversity of species and the multifunctionality of ecosystem processes.
Consumers and Ecosystem Multifunctionality.
In our field experiment, average multifunctionality increased as consumers were added (Fig. 2D and Fig. S1D), and having more consumers present had a strong, positive effect on the number of functions maintained above multifunctionality thresholds (Fig. 3). These findings reveal that the dominant salt marsh consumer assemblage made up of species from three different phyla exhibited high functional diversity (i.e., each consumer increases a different ecosystem function) and low functional redundancy (i.e., each function is controlled by no more than two consumers) (Fig. 1 A–C). A few, abundant species can maintain high levels of ecosystem multifunctionality, whereas the loss of any of these species has large, negative effects on overall functioning (Fig. S2), a result consistent with multifunctionality experiments conducted in high plant diversity systems, which also found that losing approximately one-third of species results in a 15–20% loss in ecosystem multifunctionality (11, 12) (Fig. 2D and Fig. S1D).
The striking differences in life history, body size, and foraging modes exhibited by these dominant, common, and phylogenetically diverse consumers of Southeastern US salt marshes likely drives the positive effect on overall functioning found from having more consumer species. The omnivorous crab creates extensive, communal burrow systems that can often extend meters along and below the marsh substrate and can house 10–15 crabs per burrow, indicating that where these crabs occur in moderate or high densities, there will likely be strong effects on hydrology via macropore construction. Their feeding behavior and preferences also correctly suggested that crabs would affect primary production, because it consumes live Spartina leaves by climbing into the canopy and clipping sections of the leaf with its claws and eats below-ground Spartina roots within their burrows (31, 32). The fungal-farming snail’s positive impact on decomposition is predicted by the fact that although adult individuals can consume live and dead plant tissue, they have a strong preference for eating dead plant material that has been infected with fungi facilitated through their own grazing and defecating activities (33–35). Finally, marsh fungus, which is ubiquitous across the marsh and makes up 95% of the microbial mass in standing dead and live plants (25, 26), has been found to affect both Spartina NPP (33) and decomposition (26) and has unique enzymes for breaking down complex plant-derived compounds (36). Because of this marked functional diversity of these most common marsh consumers (e.g., belowground vs. aboveground grazing, preference of live vs. dead plant grazing, different capabilities in breaking down large vs. small plant tissues, burrowing vs. climbing for refuge), our findings indicate that maintaining high levels of simultaneous functioning in ecosystems is likely determined by not just having more species (10–12), but by having more species with life histories favoring the maximization of different functions.
In this study, we thus found that strong cross-kingdom effects were likely largely responsible for maximizing overall ecosystem performance. This result indicates that the evolutionary distinctions between marsh species may play a key role in generating the radically different life history characteristics that drive differential impacts on disparate functions. These results, combined with recent experimental evidence indicating that evolutionarily diverse plant assemblages greatly enhances primary productivity (37), support the growing theory that phylogenetic and functional differences within communities may be among the most important mechanisms driving the effect of species diversity on increased functioning and community stability (24). We suggest that, where possible, future work manipulate both diversity and phylogenetic (or functional) distance to determine which has the stronger effect on ecosystem functioning.
When species known to play major but different roles in a community are lost, we found that our ability to understand the consequences of cumulative losses depends on whether more than one function is examined. Specifically, incorporating multiple-, instead of only single-, function impact assessments into our study led to a clearer understanding of the role of consumers in regulating ecosystem processes. Our findings highlight that if multifunctionality responses are not calculated in studies that measure ecosystem functions, the effect of species loss on overall ecosystem functioning can be underestimated or missed entirely. When measuring effects on single ecosystem functions, we found that adding more salt marsh consumers only enhanced one measured function (i.e., decomposition; Table 1, Fig. 2 A–C, and Fig. S1 A–C). Interpreting these single function responses alone, as is the norm for many past studies, would have led us to the conclusion that having multiple consumers, in general, was not important in overall marsh functioning. However, when we assessed ecosystem function through integrated measurement of simultaneously occurring functions, we found that the ability for a consumer assemblage to maintain multifunctionality was positively correlated with the addition of species (Figs. 2 and 3 and Fig. S1D). Additionally, only fully intact consumer assemblages consistently maintained all functions above the threshold (Fig. S2). By assessing effects on integrated measurements of processing, we were able to more accurately detect and predict the overall net effect of species extinctions on ecosystem functioning. Thus, if we are to manage ecosystems and understand the consequences of species loss, we should pay added attention to both the functional diversity of species and to the multiple, different ecosystem processes they influence.
Consumer Regulation of Coastal Wetland Ecosystem Function.
Our results have important implications for understanding process regulation and structure in economically important coastal wetlands. In salt marshes, the link between consumers and ecosystem functioning has been well established through an emphasis on top-down grazer control of marsh plant production (16, 31, 32, 38, 39). By incorporating other important marsh functions (i.e., decomposition, infiltration) and simultaneous functioning, this study greatly expands on our integrative understanding of the role played by marsh consumers in controlling other key processes. Here, we find that not only do individual consumers drive single marsh functions that are diverse (from hydrology to decomposition), but that their combined presence results in maximized rates of marsh multifunctionality. Because marine predators exert strong control over marsh community structure through trophic cascades (16) and predator diversity has been shown to be key in regulating marsh grazer impacts (40), we predict that functional and phylogenetic diversity across all trophic levels is essential in maintaining salt marsh multifunctionality. More generally, these results call for a greatly expanded line of research in coastal wetland ecology, long dominated by a bottom-up control perspective on ecosystem processes (41), that investigates linkages between community structure (e.g., biodiversity at all trophic levels) and ecosystem-level processes (e.g., shoreline stabilization, carbon sequestration, infiltration, nutrient cycling, and nursery production) by expanding the focus on plant–microbe assemblages to include food–web and indirect interactions generated by consumers. For example, future studies should investigate how consumer diversity impacts on multifunctionality vary with consumer density. At natural densities, we expect that consumer impacts on overall salt marsh functioning will be positive, as found in this study. However, at extreme high densities, as has been observed in areas devoid of marine predators or in areas stressed by drought, high densities of marsh grazers (crabs, snails, and fungi) can overwhelm primary producers and, at times, lead to permanent ecosystem loss with concomitant declines in ecosystem function (32, 42–44). In this case, density effects can trump any positive effects that arise because of the presence of phylogenetically or functionally diverse consumers.
Incorporating Microbes into Food Web Function.
Our work also reveals that incorporating microbes is important to further our understanding of the mechanistic relationship between species extinctions and the functioning of natural ecosystems. In this study, microbial consumers contributed to the positive relationship between multifunctionality and consumer richness and, without their inclusion, the importance of an intact consumer assemblage would have been underestimated, because fungal removal affected two ecosystem functions (i.e., decomposition and primary production) and multifunctionality (Fig. 1 A, B, and D). Our observation that fungi is critical to and enhances decomposition in salt marshes is a finding that is supported by a number of past studies (25, 26, 45). The positive effect of fungi on marsh NPP, as opposed to a negative (38) or no (46) effect seen in other studies, probably came about for two reasons: Fungi reduced dead plants and leaf litter in experimental plots that can shade marsh plants and suppress their growth (47), and snails that facilitate their negative effect on plants did not themselves have a significant effect on marsh plant growth (Fig. 1B), likely because their strong, suppressive impacts on plant growth primarily emerge under intense drought stress or increased densities during predator release (16). Combined, the dual enhancement of both decomposition and plant growth by fungi led to microbial presence being key for increased multifunctionality.
Although it is well known that microbes impact key ecosystem processes (48–50) and the probability of sustaining multiple ecosystem functions has been shown to increase with microbial species richness (51), our study experimentally includes microbial consumers with multiple animal consumers in a species loss-ecosystem function context. In this study, the microbial consumer had a positive effect on ecosystem functioning, but the inclusion of other types of microbes in other systems such as pathogens or parasites could also influence multifunctionality in different ways. Because microbial consumers are abundant, ubiquitous, and control diverse and multiple functions, they must be included within the greater, macroconsumer assemblage to gain an accurate understanding of the biodiversity–ecosystem function relationship and impacts of trophic feedbacks on overall food web functioning.
Methods
Study System.
Field work was carried out in a salt marsh located within the Sapelo Island National Estuarine Research Reserve (National Oceanic and Atmospheric Administration) on Sapelo Island, GA (31°25′22.81′′ N, 81°17′30.10′′ W). S. alterniflora dominates the plant community at this site, which is flooded twice daily by the tides. The field experiment was conducted in an intermediate height S. alterniflora zone (typically ∼40- to 90-cm-tall plants, flooded by ∼80% of tides) where the invertebrate consumers, the purple marsh crab, S. reticulatum, and periwinkle snail, L. irrorata, are widely distributed and abundant. S. reticulatum is a burrowing, omnivorous marsh crab that is known to consume above-ground and below-ground plant material (31, 32), whereas L. irrorata can both consume live plant tissue as well as actively farm and consume fungus on Spartina grass blades (33). The filamentous ascomycetes fungus (genera Phaesophaeria and Mycosphaerella) can occupy both live and dead plant leaves and dominates the microbial community on Spartina grass blades (25, 26). This fungus has been shown in a prior study to be ubiquitous in this and other salt marshes on Sapelo Island and can alter primary production and decomposition rates (25, 33).
Experiment.
The experiment was run for 8 mo from May to December 2011. We manipulated the presence and absence of all three species in a factorial design that yielded eight treatments and comprised four levels of diversity: three consumers (crabs + snails + fungus), two consumers (crabs + snails, crabs + fungus, snails + fungus), one consumer (crabs or snails or fungus), and no consumers. Sixty-four plots were selected (mean Spartina density: 120.8 ± 6.2 stems per m2. Treatments consisted of replicated (n = 8) 1-m2 roofless cages constructed out of 106-cm-tall aluminum flashing. To ensure that the cages did not affect tidal water flow, we poked small holes in the flashing with a screwdriver near the marsh surface to allow water to drain. To deter crabs from burrowing out of cages, we sunk aluminum flashing 35 cm into the marsh. To deter snail escape, we painted the flashing with a band of antifouling paint (Rust-Oleum Boat Bottom Antifouling Paint; Rust-Oleum). To reduce fungal biomass in fungal removal treatments, we sprayed Spartina stems with systemic fungicide (Daconil Ultrex Turf Care with Chlorothanla; Zeneca) mixed with tap water every 5 d (33, 38), which has not been found to affect growth of Spartina plants in longer-term experiments (33, 38). To control for possible positive effects of fungicide application, we sprayed an equivalent amount of tap water every 5 d in plots with ambient fungus levels. Negative effects of fungicide on the other microbes were likely unimportant in overall functioning, because ascomycete fungi make up 95% of the microbial community by mass (<5% of the community is bacteria) (25, 26). At the end of the experiment, we used ergosterol proxy techniques to determine the effect of fungicide on fungal biomass (26) and found fungicide application reduced fungal biomass by 60% (P = 0.04; Fig. S3). Although Daconil can cause animal mortality, findings from this and other studies (33, 38) found no negative effect of fungicide on crabs or snails because fungicide was applied when marsh animals were inactive (i.e., middle of a sunny day, low tide).
In each plot, the presence or absence of each consumer was manipulated and density was kept constant. We collected adult crabs (2.5–3.5 cm carapace width) and adult snails (8–12 mm shell height) from nearby marshes and stocked cages with naturally high densities observed at our field site (10 crabs per m2, 500 snails per m2) (34). Densities were monitored once a week, and crabs and snails were either added or removed as necessary to maintain treatment integrity.
Ecosystem Function 1: NPP.
To determine the effect of experimental consumer variety on NPP, net Spartina production was estimated by measuring change in live aboveground plant mass from the beginning to end of the experiment. In May when the cages were deployed, we measured stem height and density in a representative 0.5-m2 plot and converted these stem height and density values to an initial standing biomass by using regressions based on plants sampled from just outside the plots. Initial conditions were nearly identical in all plots (mean Spartina biomass: 264.3 ± 9.0 g/m2). Inside each plot, we established a permanent 0.5-m2 quadrat used to monitor live and dead Spartina stem density every month. At the conclusion of the experiment, all Spartina stems remaining in a representative 0.5-m2 plot were harvested, rinsed in water, separated into live and dead stems, dried at 60 °C in an oven for 2 d, and weighed. NPP was estimated by calculating the change in biomass (i.e., live stems only) from beginning to end of the experiment (where a value below zero indicates biomass was lost).
Ecosystem Function 2: Decomposition Rate.
We quantified the effect of consumer variety on marsh decomposition rate by deploying a plug consisting of three dead Spartina stems zip tied to a plastic flag post. In June 2011, standing dead Spartina stems were collected from a nearby marsh, rinsed, and dried for 24 h at 60 °C before taking an initial mass. Each plug weighed 7.0 ± 0.2 g initially and was placed in the middle of each plot for 30 d. After 30 d, the remaining grass was retrieved from the plot, rinsed, and dried at 60 °C for 24 h. The dry stems were weighed and mass lost (grams) was calculated. A new plug was placed in each plot, and the procedure was repeated at the beginning of July and August. Mean percent biomass lost per month was calculated across three sampling periods (June, July, and August).
Ecosystem Function 3: Infiltration Rate Measurement.
We quantified the effect of consumer variety on marsh infiltration at the conclusion of the experiment by using a double-ring infiltrometer. Two hours after a high tide, we placed a 1.5-L ring in a representative area, filled it with 1 L of water from a nearby tidal creek, and measured the time required for the water to drain out of the ring (L/min). This measurement was repeated in four plots for each of the eight treatments.
Assessing Multifunctionality.
To assess whether snail, crab, and fungi consumers differed in their ability to perform all measured functions simultaneously, we calculated an average multifunctionality index for each treatment. This method is a simple technique involving averaging standardized values of multiple functions into a single index (27, 52). For each of the three functions, we used a “standardization by maximum observed value” approach (13, 27) where we defined maximum functioning as the mean of the highest three values from all 64 plots in the experiment for each function, giving us one maximum for each function regardless of treatment. Using this maximum, plot data were recorded as the percent of that maximum for each function, creating a scaled “percent functioning” value for each individual plot. To calculate percent functioning for NPP, we added one additional step because negative net primary productivity values also would yield negative percent function. To obtain a percent functioning value between 0 and 1, we multiplied the measured NPP value from each plot by −1, and added the minimum observed value from all plots. By adding this minimum observed value to all NPP measurements, the new lowest value in the dataset (previously a negative number) becomes 0 and dividing this number by the maximum will produce a percent functioning value between 0 and 1 (13, 52). After standardizing all three functions into “percent functioning” values, we calculated the geometric mean of these three percentages to obtain a multifunctionality index for each plot, where the highest multifunctional plots will have the highest average percent functioning across all functions relative to the maximum multifunction achieved for a single plot (see refs. 13, 51, and 53 for a similar approach). Average multifunctionality calculations are useful because using this response variable can collapse complex data into a single metric that estimates the mean value of functions achieved in any given plot or assemblage (52). Average multifunctionality calculations are especially informative when multifunctionality is high, indicating that many functions are achieving high levels of performance. However, at intermediate values, it is difficult to differentiate between multiple functions all performing at intermediate levels and some performing at high values while others perform at low values. Because of this reason, we also used the multifunctionality threshold approach to evaluate whether multiple functions are simultaneously performing at high levels (52).
To more directly assess the effect of species loss on simultaneous functioning, we used a threshold based approach (11, 12, 52). This approach evaluates the ability for experimental communities to perform multiple functions simultaneously at high levels in the presence of any tradeoffs. Here we selected a multifunctionality threshold of 50% of the observed maximum of the top three plots. On average, one species is needed to keep a given process at ∼60% of its maximum rate (2) thus, using a 50% functioning threshold ensures that we can separate the effects of diversity loss from that of individual species effects, as we would expect a community with “adequate” functioning to be able to maintain multiple functions at this level (see ref. 12 for similar approach). For each plot, we recorded three values of 1 or 0, where 1 represented a function maintained over the 50% threshold and 0 represented a function maintained under this threshold. Then we summed these for each plot to get a number ranging between 0 and 3, where 3 indicated that the plot was able to maintain all functions over the threshold. To ensure species richness effects on multifunctionality were not specific to the threshold chosen, we tested thresholds ranging from 30% to 80% functioning, regarded as the most robust test for the effects of diversity on multifunctionality (52), and found similar results (i.e., the ability for a community to maintain thresholds increases significantly with species number).
Statistical Analysis.
Data were analyzed with JMP 9.0 (SAS Institute 2010). Differences in NPP, decomposition rate, and percolation rates were assessed with three-way (crab × snail × fungus) ANOVA with Tukey’s Honestly Significant Difference test used for post hoc analysis. To separate the effects of diversity loss on ecosystem functioning, we constructed one-way ANOVAs by using number of consumers as a predictor and functions as responses.
Supplementary Material
Acknowledgments
We thank J. Griffin, C. Angelini, C. Osenberg, T. Palmer, J. Byrnes, and three anonymous reviewers for comments on earlier versions of this manuscript. We also thank S. Buhler, E. Monaco, and D. Abbey for dedicated work in the field. This work was mainly supported through a National Science Foundation (NSF) CAREER Grant [biological oceanography (BIO-OCE)] awarded to B.R.S., and in part by the National Oceanic and Atmospheric Association through the Sapelo Island National Estuarine Research Reserve System Graduate Research Fellowship awarded to M.J.S.H. and by the University of Florida (University Scholars Program awarded to M.J.S.H.). This work is part of the Georgia Coastal Ecosystems Long Term Ecological Research program (NSF OCE06-20959), and is contribution number 1034 from the University of Georgia Marine Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312317110/-/DCSupplemental.
References
- 1.Balvanera P, et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett. 2006;9(10):1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443(7114):989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 4.Hooper D, Iii FC, Ewel J. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 5.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477(7363):199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 6.Stachowicz JJ, Bruno JF, Duffy JE. Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst. 2007;38:739–766. [Google Scholar]
- 7.Tilman D, Reich PB, Isbell F. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc Natl Acad Sci USA. 2012;109(26):10394–10397. doi: 10.1073/pnas.1208240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loreau M, et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science. 2001;294(5543):804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 9. MEA [Millennium Ecosystem Assessment] (2005) Ecosystems and Human Well-Being: Current State and Trends. Coastal Systems (Island, Washington)
- 10.Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448(7150):188–190. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- 11.Zavaleta ES, Pasari JR, Hulvey KB, Tilman GD. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc Natl Acad Sci USA. 2010;107(4):1443–1446. doi: 10.1073/pnas.0906829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamfeldt L, Hillebrand H, Jonsson PR. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology. 2008;89(5):1223–1231. doi: 10.1890/06-2091.1. [DOI] [PubMed] [Google Scholar]
- 13.Maestre FT, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335(6065):214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer N, Reich PB, Isbell F. Decomposer diversity and identity influence plant diversity effects on ecosystem functioning. Ecology. 2012;93(10):2227–2240. doi: 10.1890/11-2266.1. [DOI] [PubMed] [Google Scholar]
- 15.Byrnes JE, Reynolds PL, Stachowicz JJ. Invasions and extinctions reshape coastal marine food webs. PLoS ONE. 2007;2(3):e295. doi: 10.1371/journal.pone.0000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silliman BR, Bertness MD. A trophic cascade regulates salt marsh primary production. Proc Natl Acad Sci USA. 2002;99(16):10500–10505. doi: 10.1073/pnas.162366599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy J, Richardson JP, Canuel EA. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol Lett. 2003;6(7):637–645. [Google Scholar]
- 18.Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294(5548):1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- 19.Paine R. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- 20.Estes J, Duggins D. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol Monogr. 1995;65:75–100. [Google Scholar]
- 21.Duffy JE. Why biodiversity is important to the functioning of real-world ecosystems. Front Ecol Environ. 2009;7:437–444. [Google Scholar]
- 22.O’Connor NE, Crowe TP. Biodiversity loss and ecosystem functioning: Distinguishing between number and identity of species. Ecology. 2005;86:1783–1796. [Google Scholar]
- 23.Kremen C. Managing ecosystem services: What do we need to know about their ecology? Ecol Lett. 2005;8(5):468–479. doi: 10.1111/j.1461-0248.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 24.Cadotte M, Dinnage R, Tilman D. Phylogenetic diversity promotes ecosystem stability. Ecology. 2012;93:223–233. [Google Scholar]
- 25.Newell S. Spore-expulsion rates and extents of blade occupation by ascomycetes of the smooth-cordgrass standing-decay system. Bot Mar. 2001;44:277–285. [Google Scholar]
- 26.Newell S. In: The Encyclopedia of Environmental Microbiology. Bitton G, editor. New York: Wiley; 2002. pp. 1394–1400. [Google Scholar]
- 27.Hooper D, Vitousek P. Effects of plant composition and diversity on nutrient cycling. Ecol Monogr. 1998;68:121–149. [Google Scholar]
- 28.Hemond H, Nuttle W. Surface infiltration in salt marshes: Theory, measurement, and biogeochemical implications. Water Resour. 1984;20:591–600. [Google Scholar]
- 29.Loreau M. Separating sampling and other effects in biodiversity experiments. Oikos. 1998;82:600–602. [Google Scholar]
- 30.Gamfeldt L, Hillebrand H. Biodiversity effects on aquatic ecosystem functioning - maturation of a new paradigm. Int Rev Hydrobiol. 2008;93:550–564. [Google Scholar]
- 31.Holdredge C, Bertness MD, Altieri AH. Role of crab herbivory in die-off of New England salt marshes. Conserv Biol. 2009;23(3):672–679. doi: 10.1111/j.1523-1739.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 32.Coverdale TC, Altieri AH, Bertness MD. Belowground herbivory increases vulnerability of New England salt marshes to die-off. Ecology. 2012;93(9):2085–2094. doi: 10.1890/12-0010.1. [DOI] [PubMed] [Google Scholar]
- 33.Silliman BR, Newell SY. Fungal farming in a snail. Proc Natl Acad Sci USA. 2003;100(26):15643–15648. doi: 10.1073/pnas.2535227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silliman B, Zieman J. Top-down control of Spartina alterniflora production by periwinkle grazing in a Virginia salt marsh. Ecology. 2001;82:2830–2845. [Google Scholar]
- 35.Bärlocher F, Newell S. Growth of the salt marsh periwinkleLittoraria irrorata on fungal and cordgrass diets. Mar Biol. 1994;114:109–114. [Google Scholar]
- 36.Bärlocher F, Newell S, Arsuffi T. Digestion of Spartina alterniflora Loisel material with and without fungal constituents by the periwinkle Littorina irrorata Say (Mollusca: Gastropoda) Journal of Experimental Marine Biology. 1989;130:45–53. [Google Scholar]
- 37.Cadotte MW. Experimental evidence that evolutionarily diverse assemblages result in higher productivity. Proc Natl Acad Sci USA. 2013;110(22):8996–9000. doi: 10.1073/pnas.1301685110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daleo P, et al. Grazer facilitation of fungal infection and the control of plant growth in south-western Atlantic salt marshes. J Ecol. 2009;97:781–787. [Google Scholar]
- 39.Alberti J, et al. Local and geographic variation in grazing intensity by herbivorous crabs in SW Atlantic salt marshes. Mar Ecol Prog Ser. 2007;349:235–243. [Google Scholar]
- 40.Griffin JN, Silliman BR. Predator diversity stabilizes and strengthens trophic control of a keystone grazer. Biol Lett. 2011;7(1):79–82. doi: 10.1098/rsbl.2010.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitsch WJ, Gosselink JG. Wetlands. New York: Van Nostrand Reinhold; 2001. [Google Scholar]
- 42.Altieri AH, Bertness MD, Coverdale TC, Herrmann NC, Angelini C. A trophic cascade triggers collapse of a salt-marsh ecosystem with intensive recreational fishing. Ecology. 2012;93(6):1402–1410. doi: 10.1890/11-1314.1. [DOI] [PubMed] [Google Scholar]
- 43.Silliman BR, McCoy MW, Angelini C, Holt R, Griffin J, van de Koppel J. Consumer fronts, global change, and runaway collapse in ecosystems. Annu Rev Ecol Evol Syst. 2013;44:503–538. [Google Scholar]
- 44.Silliman BR, van de Koppel J, Bertness MD, Stanton LE, Mendelssohn IA. Drought, snails, and large-scale die-off of southern U.S. salt marshes. Science. 2005;310(5755):1803–1806. doi: 10.1126/science.1118229. [DOI] [PubMed] [Google Scholar]
- 45.Newell S, Fallon R, Miller J. Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Mar Biol. 1989;481:471–481. [Google Scholar]
- 46.Sala N, Bertness M, Silliman B. The dynamics of bottom–up and top–down control in a New England salt marsh. Oikos. 2008;117:1050–1056. [Google Scholar]
- 47.Holdredge C, Bertness MD. Litter legacy increases the competitive advantage of invasive Phragmites australis in New England wetlands. Biol Invasions. 2010;13:423–433. [Google Scholar]
- 48.Peay KG, Dickie IA, Wardle DA, Bellingham PJ, Fukami T. Rat invasion of islands alters fungal community structure, but not wood decomposition rates. Oikos. 2012;122(2):258–264. [Google Scholar]
- 49.Eisenhauer N, Scheu S, Jousset A. Bacterial diversity stabilizes community productivity. PLoS ONE. 2012;7(3):e34517. doi: 10.1371/journal.pone.0034517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell T, Newman JA, Silverman BW, Turner SL, Lilley AK. The contribution of species richness and composition to bacterial services. Nature. 2005;436(7054):1157–1160. doi: 10.1038/nature03891. [DOI] [PubMed] [Google Scholar]
- 51.Maestre FT, Castillo-Monroy AP, Bowker MA, Ochoa-Hueso R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J Ecol. 2012;100:317–330. [Google Scholar]
- 52.Byrnes J, et al. 2013. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. arXiv:1305.1985v1.
- 53.Mouillot D, Villéger S, Scherer-Lorenzen M, Mason NWH. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE. 2011;6(3):e17476. doi: 10.1371/journal.pone.0017476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.