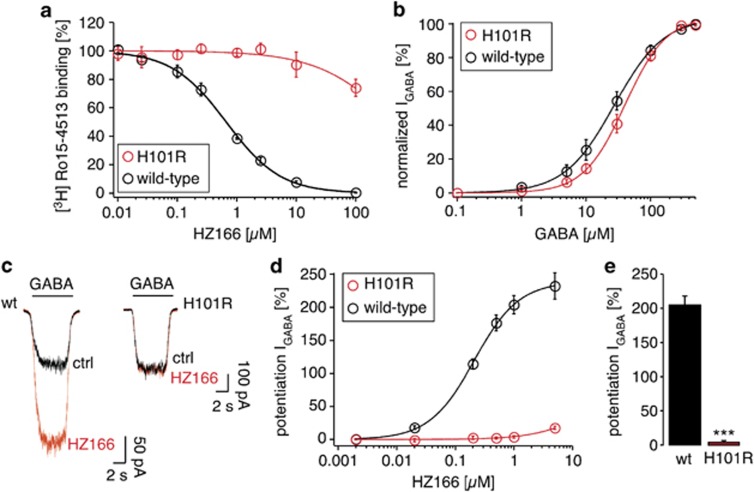
Figure 2.
HZ166 binding properties to recombinant wild-type and α2(H101R)β3γ2-GABAARs and potentiation by HZ166. (a) Binding affinity of HZ166 to wild-type and α2(H101R)β3γ2 point-mutated GABAARs determined by [3H] Ro15-4513 displacement. Ki in wild-type GABAARs was 221±22 nM (mean±SEM, n=4). (b–e) Electrophysiological analyses (e). (b) Activation of wild-type and α2(H101R)β3γ2 GABAARs exhibited similar dependence on GABA concentration. EC50 was 27±1 μM (mean±SEM, n=8) and 40±2 μM (n=7) for wild-type and α2(H101R)β3γ2 point-mutated GABAARs, respectively. Hill coefficients were 1.2±0.03 and 1.4±0.07, and maximum currents were 3.6±0.4 nA and 3.5±0.7 nA. (c–e) Potentiation by HZ166. (c) Example of current traces evoked by GABA (EC5=5 μM) in the presence or absence of HZ166 (1 μM). (d) Concentration–response curves of wild-type and α2(H101R)β3γ2 point-mutated GABAARs for HZ166. EC50 of HZ166 for wild-type GABAARs: 217±20 nM. (e) Statistical comparison of potentiation by HZ166 (1 μM). ***P<0.001 (wild-type vs point-mutated receptors), unpaired Student's t-test.