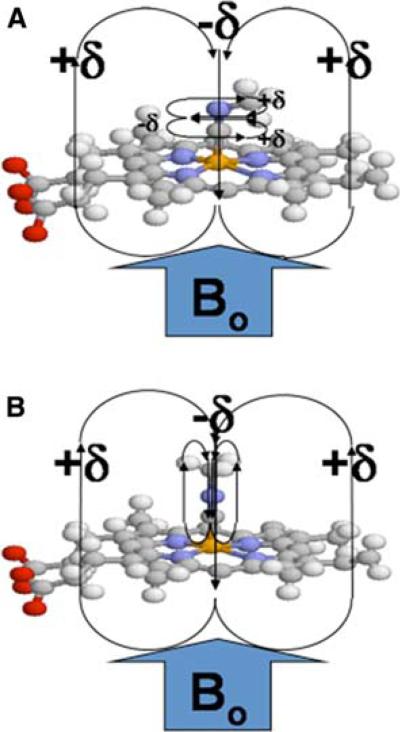
Fig. 1.
Methyl isocyanide bound to the heme iron. Schematic representation of methyl isocyanide bound to heme with (a) an iron-carbon bond order of two (“bent”) and (b) an iron-carbon bond order of one (“linear”). The heme pyrrole rings, along with the CN triple (or double) bond, produce a highly anisotropic environment that can produce large chemical shift perturbations for the labeled methyl group