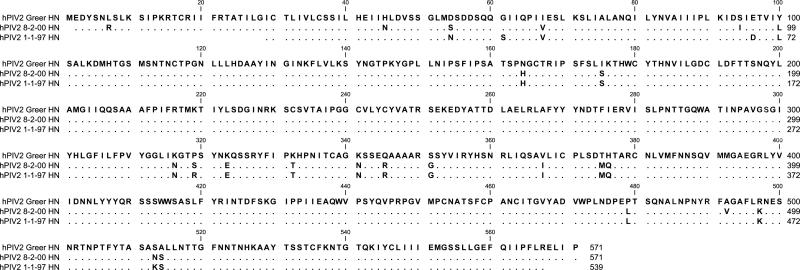
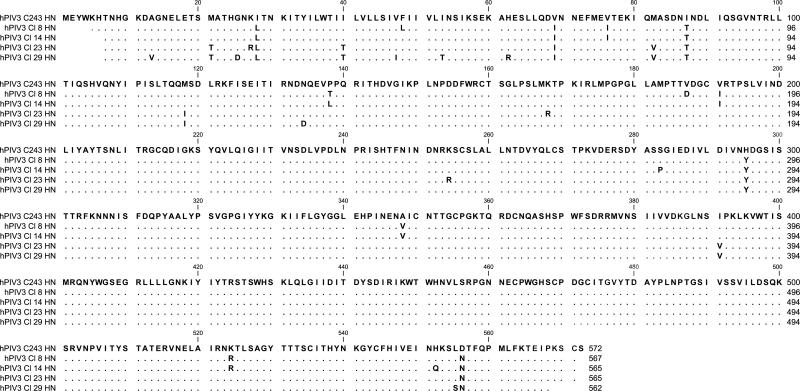
Figure 1. hPIV-2 and hPIV-3 protein sequences.
Sanger sequencing was conducted to identify HN sequences for each primary virus isolate. Predicted amino acid sequences are aligned to the templates of Greer for hPIV-2 (1A) and C243 for hPIV-3 (1B). A blank position indicates lack of sequence information. A dot indicates a match with the template, and a single-letter code indicates an amino acid substitution.
Methods. Viral RNA was purified using a Qiagen QIAmp Viral RNA Mini Kit (Cat# 52904), and RT-PCR was conducted with virus sequence-specific primers (synthesized by Integrated DNA Technologies) and a RNA One-step PCR Kit from TaKaRa Bio, Inc. (Cat# RR024). Sanger sequencing was then performed at the Hartwell Center of St. Jude Children's Research Hospital using additional virus sequence-specific primers. For analyses, individual sequences were imported into DNA Lasergene 7 SeqMan software and aligned to create a consensus contig. Contigs were translated and aligned using CLC Main Workbench 5 software.