Table 1.
Angularly Substituted Indenoisoquinolines
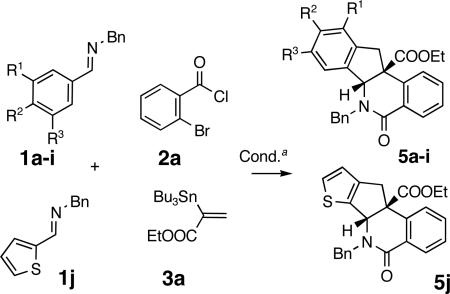
| |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | prdtb | yieldc (%) |
| 1 | H | OMe | H | 5a | 75 (71)d |
| 2 | H | Me | H | 5b | 69 |
| 3 | H | H | H | 5c | 67 (17)d |
| 4 | H | Cl | H | 5d | 49 (11)d |
| 5 | H | COOMe | H | 5e | 38 |
| 6 | H | OMe | OMe | 5f | 77 |
| 7 | H | -OCH2CH2O- | 5ge | 71 | |
| 8 | OMe | OMe | OMe | 5h | 64 |
| 9 | H | NMe2 | H | 5i | 59 |
| 10 | thiophene-2-yl | 5j | 74 | ||
aMethod A: was used for all the entries: (i) CuCl (20%), MeCN/CH2Cl2, 45 °C, 6 h, imine : acyl chloride : stannane = 1.0 : 1.2 : 1.5 (mol); (ii) aqueous KF, filtration, evaporation; (iii) Pd(OAc)2 (5%), NaOAc (1.0 equiv), DMF, 120 °C, 24 h.
With one exception, a single diastereomer was isolated.
Isolated yield of heterocycles 5 obtained via Method A calculated per imine as the limiting reagent.
Yield of heterocycle 5 obtained by Method B is given in parentheses. Method B: same as Method A, but substituting Na2CO3 (1.0 equiv)/n-Bu4NCl (1.0 equiv) for NaOAc.
Product was isolated as a 4 : 1 mixture of diastereomers (by 1H NMR), the major diastereomer is shown.
