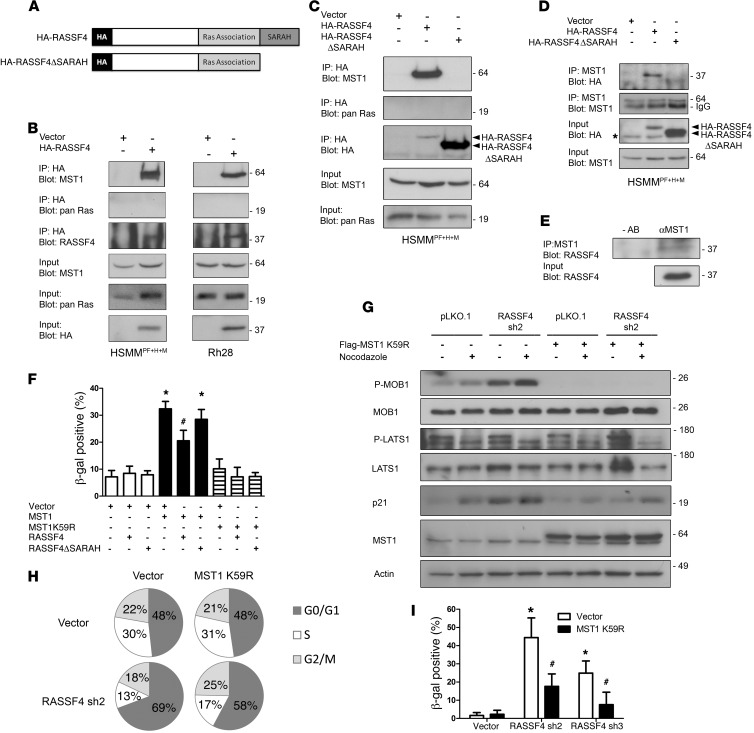
Figure 5. RASSF4 regulates MST1 to inhibit the Hippo pathway in aRMS.
(A) Domain architecture of HA-RASSF4 constructs. (B) RASSF4 associated with MST1 in aRMS cells. Anti-HA immunoprecipitates from aRMS cells expressing HA-RASSF4 or an empty vector were examined for coprecipitation of MST1 or pan H-, K-, and N-Ras by immunoblotting. (C) RASSF4-MST1 association was dependent on the RASSF4 SARAH domain. Anti-HA immunoprecipitates from HSMMPF+H+M cells expressing HA-RASSF4, HA-RASSF4ΔSARAH, or vector were used to examine the association with MST1 or pan Ras by immunoblotting. These results were confirmed by immunopurifying endogenous MST1 and by blotting for HA-RASSF4, HA-RASSF4ΔSARAH (D, top), or endogenous RASSF4 (E). (F) Exogenous MST1 expression induced aRMS cell senescence, which was partially inhibited by HA-RASSF4 expression. Error bars represent SD. *P < 0.0001 compared with vector expressed alone. #P = 0.0005 compared with MST1-expressing cells. (G) RASSF4 suppressed MST1 signaling to MOB1. HSMMPF+H+M cells expressing vector or MST1K59R, vector, or RASSF4 shRNA were cultured in the presence or absence of nocodazole. Protein lysates from these cells were analyzed by immunoblotting. (H) MST1 K59R partially blocked G0/G1 accumulation in RASSF4-deficient cells as measured by cell cycle analysis. (I) MST1K59R prevented senescence induction caused by RASSF4 loss as measured by β-gal assay. *P < 0.00005 compared with vector control cells; #P < 0.005 compared with cells with RASSF4 knockdown alone.