Figure 1. A schematic of the UPS.
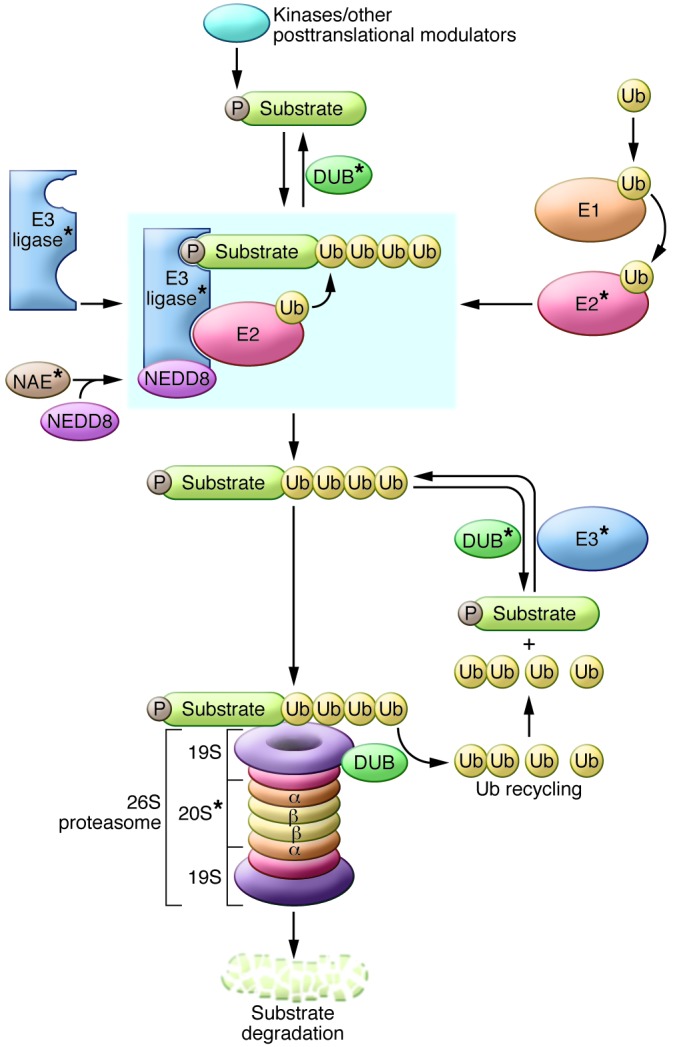
Ub is loaded onto the E1 activating enzyme in an ATP-dependent fashion and then transferred to an E2 Ub-conjugating enzyme. For the case of cullin-RING E3 ligases, the E3 is primed by neddylation by the NEDD8-activating enzyme (NAE). E3 ligases may be single proteins or multi-subunit enzyme complexes that mediate Ub transfer from an E2 conjugating enzyme to the substrate through interaction of a degron motif (usually a posttranslationally modified molecular recognition signature such as phosphorylation [P]) within the substrate and the binding domain of the E3. Ub monomers are covalently added to the substrate protein, and the polyubiquitinated protein is recognized and bound by the 19S subunit and degraded by the 20S subunit of the 26S proteasome. DUB enzymes are capable of “rescuing” Ub substrates from degradation, while other DUBs mediate cleavage and recycling of the Ub monomers. Components that are potential therapeutic targets are indicated by asterisks.
