Abstract
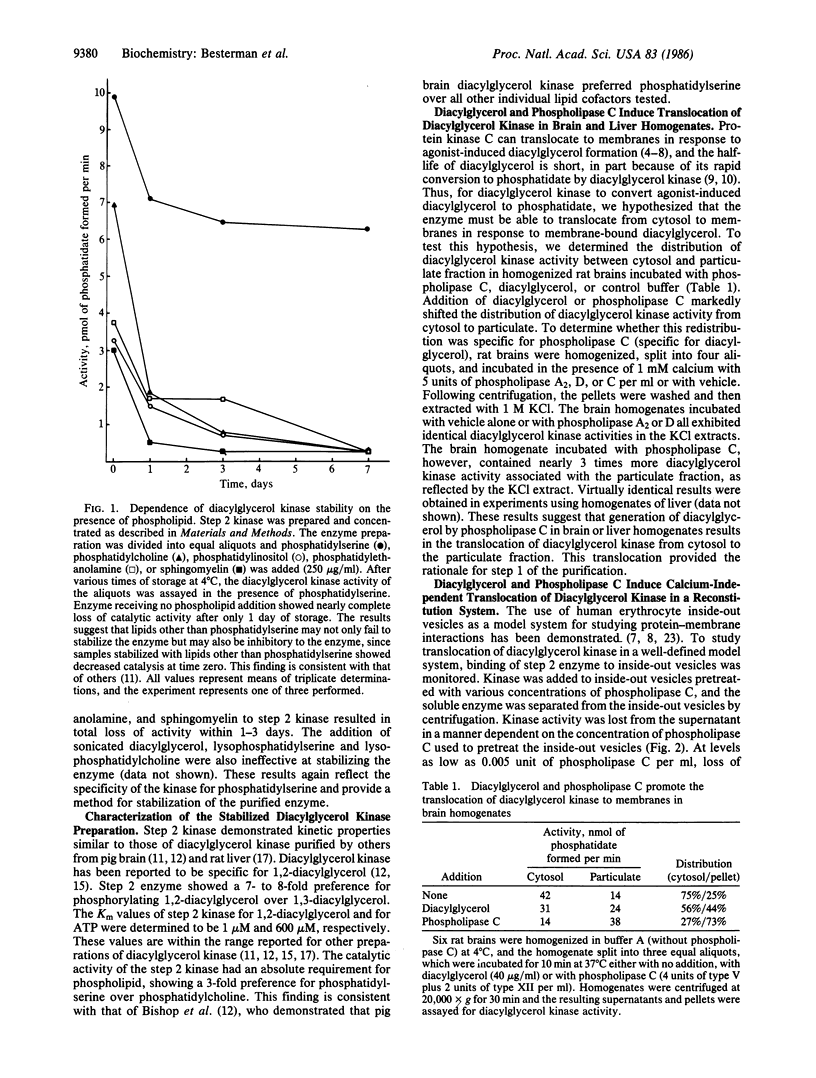
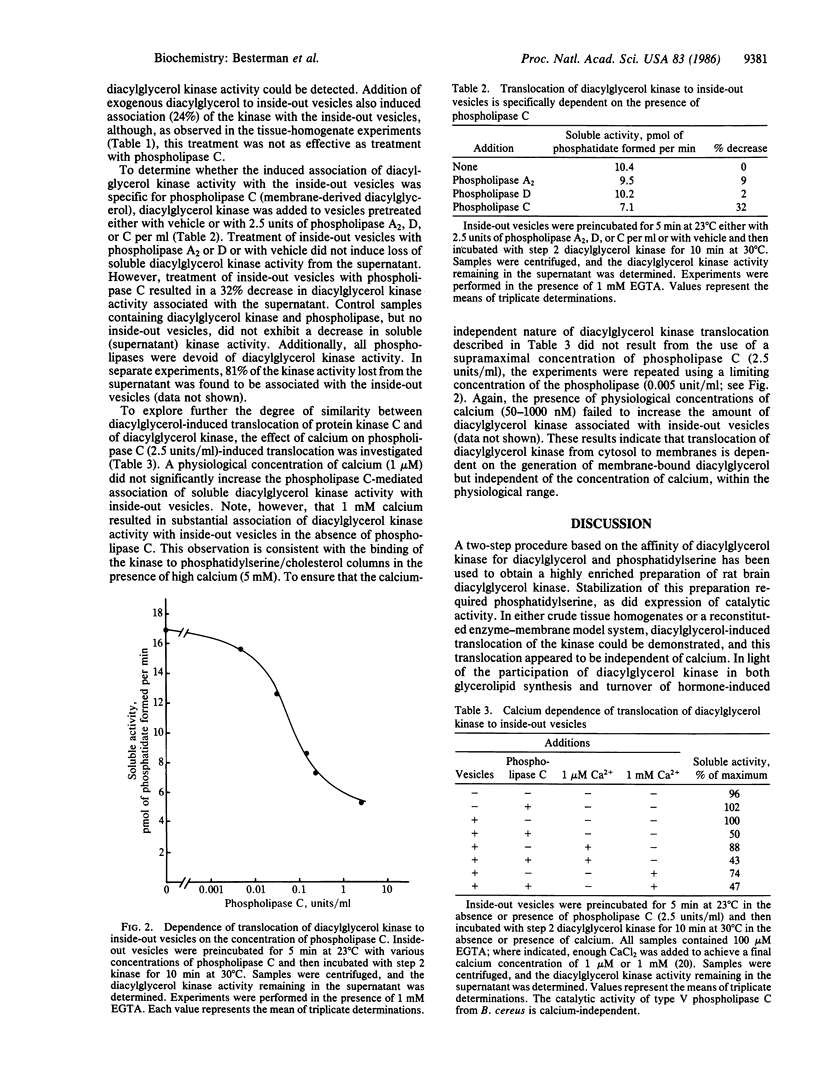
Diacylglycerol-induced translocation of diacylglycerol kinase (ATP:1,2-diacylglycerol 3-phosphotransferase, EC 2.7.1.107) from the soluble to the membrane-bound compartments was demonstrated both in crude tissue homogenates and in a reconstituted enzyme-membrane model system. In homogenates of either rat brain or liver, incubation with diacylglycerol or phospholipase C, but not phospholipase A2 or phospholipase D, resulted in the translocation of diacylglycerol kinase activity from the soluble to the particulate fraction. This observation formed the basis for the first step in a two-step purification of diacylglycerol kinase. Enzyme extracted in 1 M salt from membranes of rat brain homogenates made in the presence of phospholipase C was purified further by affinity chromatography on a column containing phosphatidylserine, diacylglycerol, and cholesterol immobilized in polyacrylamide. This step yielded an enzyme preparation (step 2 enzyme) that was 500- to 750-fold purified (relative to the tissue homogenate) and required phosphatidylserine for stability. All other lipids tested failed to stabilize the enzyme. The properties of the enzyme preparation were similar to those of mammalian diacylglycerol kinases described by others. Reconstitution experiments showed that the soluble step 2 enzyme bound to inside-out vesicles of human erythrocytes only in the presence of diacylglycerol or phospholipase C but not phospholipase A2 or D. Redistribution of the kinase from soluble to vesicle-bound forms occurred rapidly and was dependent on the concentration of phospholipase C used to treat the vesicles. Physiological concentrations of calcium (50-1000 nM) did not enhance the phospholipase C-mediated translocation of the kinase. Thus, diacylglycerol kinase can translocate from cytosol to membranes in a manner dependent on the content of membrane-bound diacylglycerol but independent of the ambient concentration of calcium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. The synthesis of phosphatidic acid from diglyceride and adenosine triphosphate in extracts of brain microsomes. J Biol Chem. 1959 Jun;234(6):1381–1386. [PubMed] [Google Scholar]
- Kanoh H., Akesson B. Properties of microsomal and soluble diacylglycerol kinase in rat liver. Eur J Biochem. 1978 Apr;85(1):225–232. doi: 10.1111/j.1432-1033.1978.tb12230.x. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Iwata T., Ono T., Suzuki T. Immunological characterization of sn-1,2-diacylglycerol and sn-2-monoacylglycerol kinase from pig brain. J Biol Chem. 1986 Apr 25;261(12):5597–5602. [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kanoh H., Ohno K. Partial purification and properties of diacylglycerol kinase from rat liver cytosol. Arch Biochem Biophys. 1981 Jun;209(1):266–275. doi: 10.1016/0003-9861(81)90280-0. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Stimulation of phosphatidic acid production in platelets precedes the formation of arachidonate and parallels the release of serotonin. Biochim Biophys Acta. 1979 May 25;573(2):394–402. doi: 10.1016/0005-2760(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Hawthorne J. N. The diglyceride kinase of rat cerebral cortex. Biochem J. 1971 Apr;122(2):171–179. doi: 10.1042/bj1220171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Sharkey N. A., Leach K. L., Blumberg P. M. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci U S A. 1984 Jan;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Wells W. W. Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J Biol Chem. 1983 Aug 10;258(15):9368–9373. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Uchida T., Filburn C. R. Affinity chromatography of protein kinase C-phorbol ester receptor on polyacrylamide-immobilized phosphatidylserine. J Biol Chem. 1984 Oct 25;259(20):12311–12314. [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]


