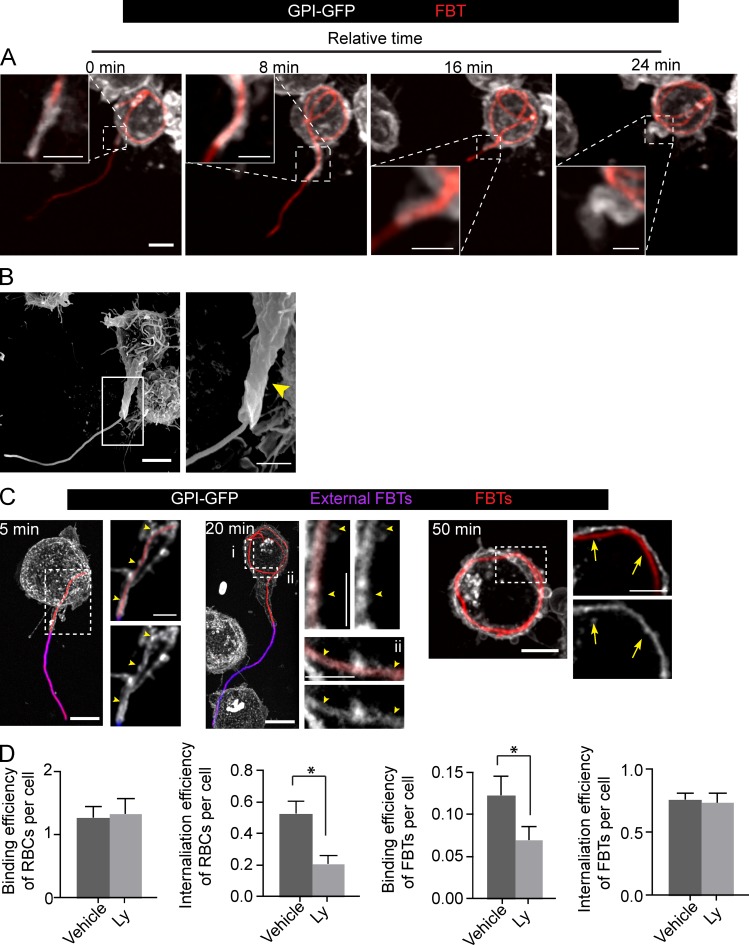
Figure 1.
Uptake of bacterial filaments occurs through a tubular phagocytic cup. (A) Phagocytosis of FBTs by GPI-GFP expressing RAW macrophage. Frames from a time-lapse sequence depict the T-PC extending and engulfing the FBT after 15 min of phagocytosis (T0). Insets show higher magnification of framed regions. (B) Scanning electron micrograph of RAW cell showing the T-PC (arrowhead) formed after 20 min of phagocytosis. (C) T-PCs remain positive for GPI until the FBTs are fully internalized. Phagocytosis was allowed to proceed to indicated times, cells fixed, and external FBTs were immunolabeled (pink). For each time point, panels on the right are magnified single planes from framed regions. GPI-GFP–positive T-PCs (arrowheads) and loss of GPI-GFP from fully internalized FBTs (arrows) are indicated. (D) PI3K activity is not required for FBT internalization. Cells pretreated with 100 µM LY294002 (Ly) for 30 min were presented with opsonized RBCs or FBTs and phagocytosis allowed to proceed for 50 min in the presence of Ly. Cells were fixed, external particles were immunolabeled, and cells permeabilized to visualize internal particles. Attachment was enumerated for random fields. Efficiency of internalization was determined by assessing the proportion of internalized particles/total particles. Data shown are means ± SEM from three independent experiments. For binding efficiency, n = 150 RAW cells and for internalization efficienty, n = 100 RBCs or 30 FBTs in each experiment. Bars: (main panels) 5 µm; (magnifications) 2.5 µm.