Figure 5.
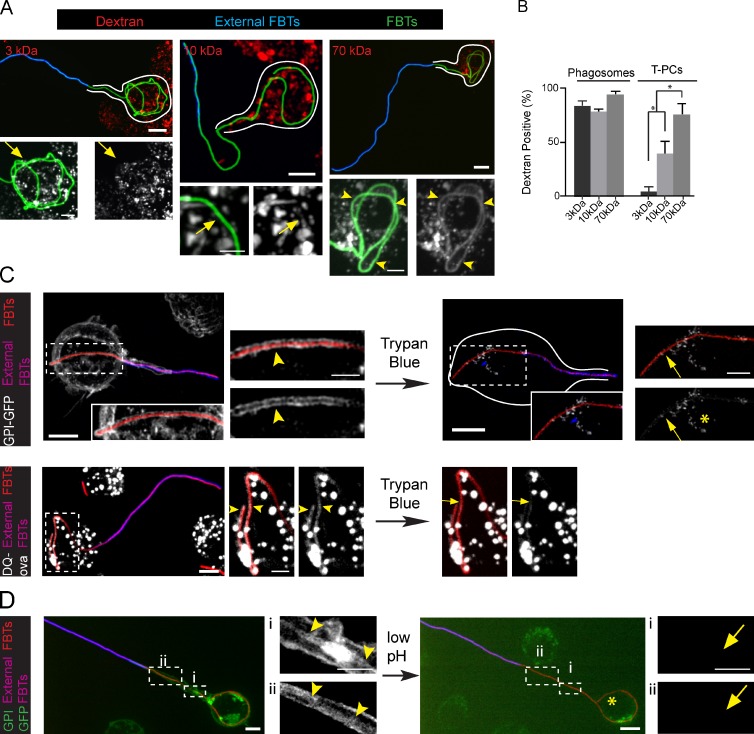
Low molecular weight solutes permeate across the T-PCs. (A) RAW cells preloaded with 3-, 10-, and 70-kD rhodamine dextrans with pulse and chase were allowed to ingest GFP-FBTs for 20 min and external FBTs were immunolabeled (blue). Top: partially internalized FBTs. White lines indicate cell boundaries. Bottom: higher magnifications of internal segments of FBTs. Dextrans are shown in white. 70-kD dextran accumulated around internal portions of FBTs (arrowheads), while 3-kD and 10-kD dextrans were absent (arrows). (B) The number of partially and fully internalized dextran-positive FBTs from A. Data shown are means ± SEM from three independent experiments; n = 50. (C) Trypan blue leaks into the T-PCs and quenches GPI-GFP (white) fluorescence from the T-PC membrane (top panels) and DQ-ova (white) from T-PC lumen (bottom panels). After 20 min of FBT phagocytosis by cells stably expressing GPI-GFP, external FBTs were labeled in the cold. Cells were moved to a precooled microscope stage and trypan blue was added to the extracellular media. Left: GPI-GFP– and DQ-ova–positive T-PCs (arrowheads). Right: GFP from T-PCs is quenched by trypan blue (arrows). Endosomes that were inaccessible to trypan blue were not quenched (asterisk). (D) T-PCs are permeable to protons. 20 min after phagocytosis of FBTs in GPI-GFP–expressing cells, external FBTs were labeled in the cold (pink). Cells were moved to a precooled microscope stage and extracellular pH was reduced to 4.0. Right: arrowheads indicate the T-PC containing GPI-GFP (white). Left: quenching of GFP from the T-PC (arrows) after reducing extracellular pH. Asterisk denotes endosomes not affected by the treatment. Bars: (main panels) 5 µm; (magnifications) 2.5 µm.