Abstract
N-Acylhomoserine lactone (AHL)-mediated quorum-sensing (QS) regulates virulence functions in plant and animal pathogens such as Agrobacterium tumefaciens and Pseudomonas aeruginosa. A chemolibrary of more than 3500 compounds was screened using two bacterial AHL-biosensors to identify QS-inhibitors (QSIs). The purity and structure of 15 QSIs selected through this screening were verified using HPLC MS/MS tools and their activity tested on the A. tumefaciens and P. aeruginosa bacterial models. The IC50 value of the identified QSIs ranged from 2.5 to 90 µg/ml, values that are in the same range as those reported for the previously identified QSI 4-nitropyridine-N-oxide (IC50 24 µg/ml). Under the tested culture conditions, most of the identified QSIs did not exhibit bacteriostatic or bactericidal activities. One third of the tested QSIs, including the plant compound hordenine and the human sexual hormone estrone, decreased the frequency of the QS-regulated horizontal transfer of the tumor-inducing (Ti) plasmid in A. tumefaciens. Hordenine, estrone as well as its structural relatives estriol and estradiol, also decreased AHL accumulation and the expression of six QS-regulated genes (lasI, lasR, lasB, rhlI, rhlR, and rhlA) in cultures of the opportunist pathogen P. aeruginosa. Moreover, the ectopic expression of the AHL-receptors RhlR and LasR of P. aeruginosa in E. coli showed that their gene-regulatory activity was affected by the QSIs. Finally, modeling of the structural interactions between the human hormones and AHL-receptors LasR of P. aeruginosa and TraR of A. tumefaciens confirmed the competitive binding capability of the human sexual hormones. This work indicates potential interferences between bacterial and eukaryotic hormonal communications.
Introduction
Bacterial populations synthesize and exchange chemical signals which coordinate and synchronize gene expression in a cell-density dependent manner. Such regulatory pathways are called quorum-sensing (QS) and involve diverse QS-signals, including N-acylhomoserine lactones (AHLs) [1]. The canonical proteins required for the synthesis of AHLs belong to the LuxI family, and those for AHL-sensing to the LuxR family [2]. The AHL-mediated QS is widespread among Proteobacteria, controlling - for instance - the expression of genes involved in bacterial virulence in animal and plant hosts, horizontal gene transfer by plasmid conjugation, as well as bacterial competitiveness in the environment through production of antibiotics [1]–[2].
Natural and synthetic compounds which alter QS signalling and thereby disrupt QS-regulated gene expression are called QS inhibitors (QSIs). Considering the central role played by QS in the expression of virulence genes in pathogenic bacteria, the search for QSIs has driven many efforts [3]. Over the past several years, numerous QSIs with diverse structures have been identified using different approaches such as the synthesis of structural analogues, experimental and virtual screening of chemo-libraries and purification of natural QSIs from diverse organisms, especially plants [3]–[5]. The natural QSIs contribute to host defense against bacteria and both natural and synthetic QSIs have been proposed as promising molecules because they may act synergistically with antibiotics to limit bacterial infection [6]–[8].
In this work, we screened a chemo-library for the presence of QSIs and validated the QSI activity of the identified compounds using two bacterial species, the plant pathogen Agrobacterium tumefaciens in which QS regulates the horizontal transfer of the tumor-inducing (Ti) plasmid, and the opportunistic pathogen Pseudomonas aeruginosa, in which QS controls the expression of virulence factors. This paper reports the identification of novel natural (hordenine) and synthetic (indoline-2-carboxamides) QSIs, and also experimentally demonstrates QSI-activity of three human sexual hormones: estrone, estriol, and estradiol.
Results
Identification of the QSIs
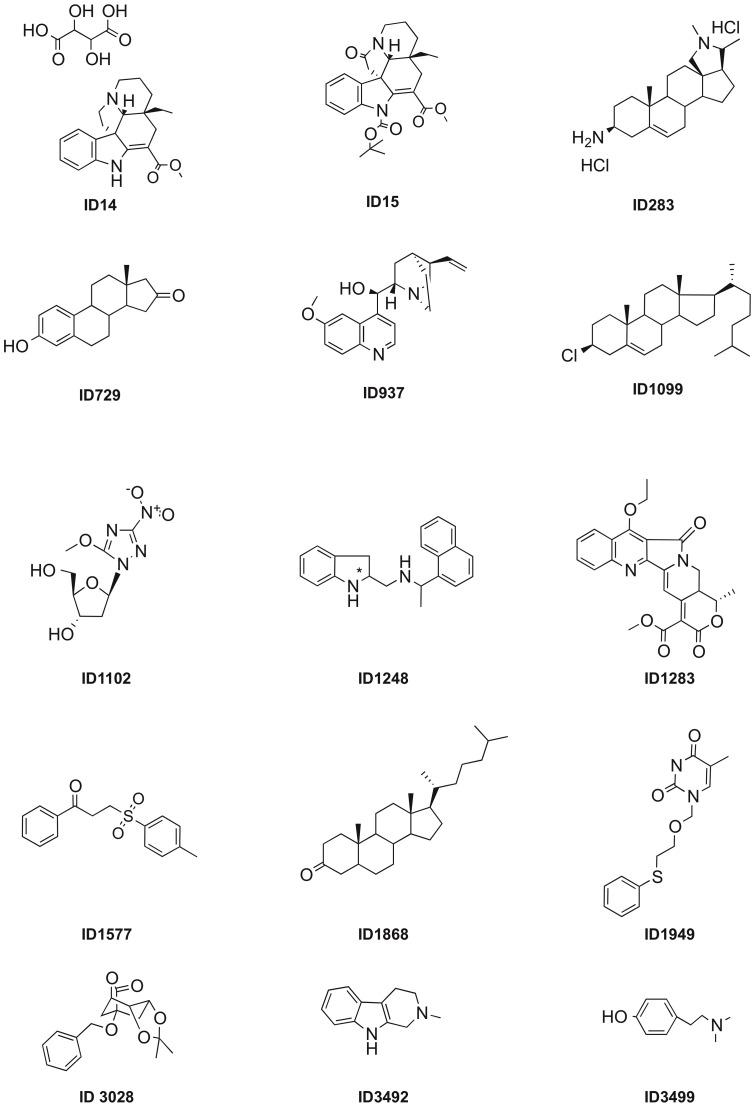
The ICSN chemical library (see materials and methods) was screened with two bacterial AHL-bioindicators, C. violaceum CV026 and A. tumefaciens NT1(pZLR4) in the presence of the appropriate AHLs. The strains and plasmids used in this study are listed in Table 1. Using C. violaceum CV026 in association with hexanoylhomoserine lactone (C6-HSL) at 0.5 µM and the tested compounds at 50 µg/ml, over 150 potential QSIs corresponding to ca. 5% of the chemical library compounds, were identified. To improve the selectivity of the screening, we reduced the concentration of the tested compounds to 5 µg/ml and used the A. tumefaciens biosensor which is sensitive to very low amounts (10 nM) of octanoylhomoserine lactone (C8-HSL). From this second screening, 25 molecules, i.e. 0.7% of the 3520 tested compounds, emerged as potent QSIs. Ten out of the 25 identified molecules (e.g. novobiocin, quinine, ochrolifuanine A and o, β-dinitro-β-methylstyrene) are already known as antimicrobial agents. They were consequently removed from this study. Hence, only 15 of the identified hits numbered 14, 15, 283, 729, 937, 1099, 1102, 1248, 1283, 1577, 1868, 1949, 3028, 3492, and 3499 were retained for further analyses (Figure 1). Compounds 14 [9]–[10], 15 [11], 1102 [12], 1283 [13], 1577 [14]–[15], 3028 [16] have previously been described, while compounds 283, 729, 1248 and 1949 are described in the experimental section. Compounds 937, 1099, 1868, 3492 and 3499 are commercially available (Sigma Aldrich and SynChem, Inc.). The 15 hits belong to different structural families such as carbazole (i.e. 15), indoline (i.e. 1248), pyridoindole (i.e. 3492), steroids (i.e. 1099, 1868) including the human sexual hormone estrone (i.e. 729), as well as the plant phenylethylamine alkaloid hordenine (i.e. 3499).
Table 1. Bacterial strains and plasmids used in this study.
| Strains and plasmids | Relevant characteristics | Reference or source |
| Agrobacterium NT1(pZLR4) | A. tumefaciens C58 derivativeexpressing traR and traG-lacZ, AHL-bioindicator | [42] |
| Agrobacterium pTi-donor | A. tumefaciens C58 derivative with pTiC58ΔaccRΔtraIKm | [45] |
| A. tumefaciens C58-00 | A. tumefaciens C58 derivative, cured of its plasmids, recipient strain | Lab collection, CNRS, Gif-sur-Yvette |
| Chromobacterium violaceum CV026 | C. violaceum ATCC 31532derivative, violacein producer, AHL-bioindicator | [41] |
| Escherichia coli JLD271 | K-12 derivativeΔlacX74sdiA271::Cam | [21] |
| Pseudomonas aeruginosa PAO1 | Wild-type | http://www.pseudomonas.med.ecu.edu/ |
| pβ01 | pQF50-derivative, PlasB-lacZ | [51] |
| pβ02 | pQF50-derivative,PrhlA-lacZ | [51] |
| pLPR1 | pLP170-derivative, PrhlI-lacZ | [52] |
| pPCS223 | pLP170-derivative, PlasI-lacZ | [52] |
| pPCS1001 | pLP170-derivative, PlasR-lacZ | [53] |
| pPCS1002 | pLP170-derivative,PrhlR-lacZ | [53] |
| pAL101 | pSB401-derivative, rhlR+rhlI::luxCDABE | [21] |
| pAL102 | pSB401-derivative, rhlI::luxCDABE | [21] |
| pAL105 | pSB401-derivative, lasR+lasI::luxCDABE | [21] |
| pAL106 | pSB401-derivative, lasI::luxCDABE | [21] |
| pTB4124 | pQF50-derivative, PaceA-lacZ | [20] |
Figure 1. Structures of the QSIs identified in the chemical library.
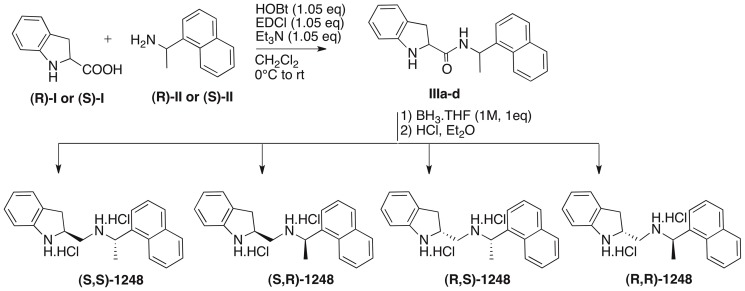
Synthesis of each diastereoisomer of the QSI ID1248
Sample ID1248 being a mixture of 4 stereoisomers, each 4 was synthesized separately and unambiguously starting from the commercially available, optically-active precursors to determine which isomer is the most active (Figure 2). Thus, (R)- and (S)-indoline-2-carboxylic acid I were each coupled with (R)- and (S)-1-(1-naphthyl)ethylamine II using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) and 1-hydroxybenzotriazole (HOBt) in dichloromethane. The carboxamide bond of each compound formed (IIIa-d) was then reduced to the amine using borane-THF complex and the products ( S,S )-1248, ( S,R )-1248, ( R,S )-1248 and ( R,R )-1248 were isolated as their hydrochloride salts.
Figure 2. Synthesis of the 4 diastereoisomers of QSI-1248.
IC50 values and bacterial toxicity of the QSIs in A. tumefaciens
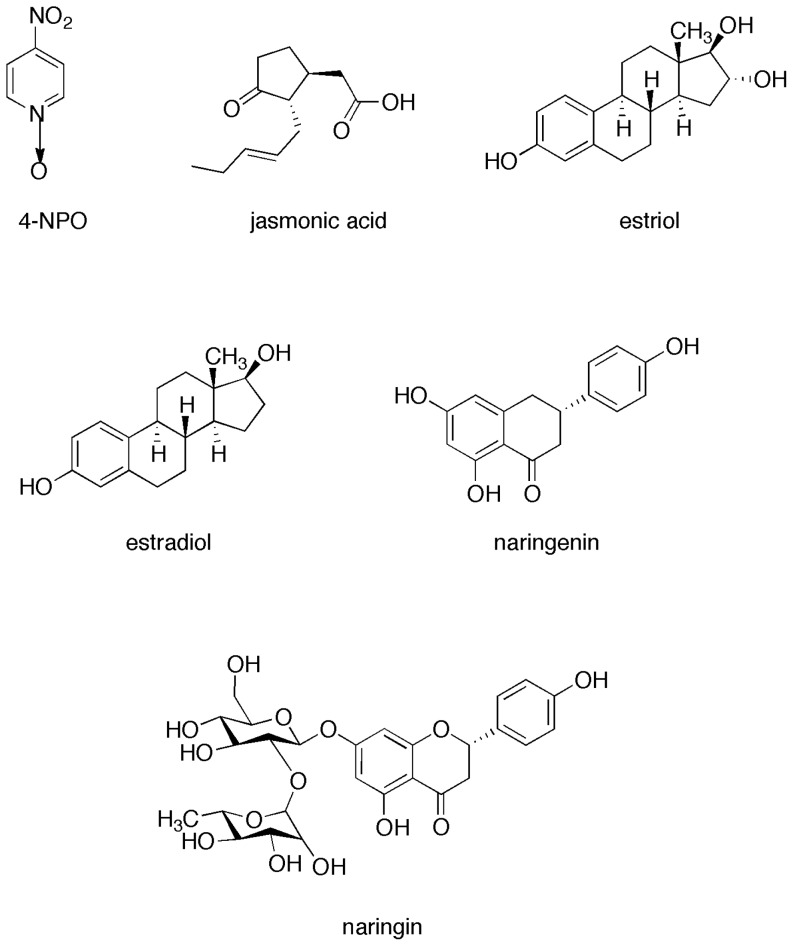
The IC50 values of the chemical library QSIs and commercial compounds (Figure 3), such as the hormones estradiol and estriol, the plant-defense signal jasmonic acid, and the QSI-reference 4-nitropyridine-N-oxide (4-NPO) already published by Rasmussen et al [17], were measured using the A. tumefaciens bioindicator that expresse the traG-lacZ reporter fusion. According to our procedure, the QSI-reference 4-NPO exhibited an IC50 of 24 µg/ml (Table 2). Compound 1577 exhibited an IC50 value (IC50 = 2.5 µg/ml) lower than that of 4-NPO. The IC50 of 10 compounds ranged between 30 and 90 µg/ml (283, 729, 1099, 1248, (S,S)-1248, (S,R)-1248, 3492, jasmonic acid, estradiol and estriol), while the other IC50 values were higher than 100 µg/ml (14, 15, 937, 1102, 1289, (R,S)-1248, (R,R)-1248 and 3499). Notably, (S,R)-1248 exhibited a QSI activity higher than that of the racemic mixture of 1248 and the other diastereoisomers. This stereoselectivity of the inhibitory activity points out the most probable occurrence of a specific interaction of the QSI with the biological target rather than to simple non-specific activity.
Figure 3. Structure of the additional QSIs and compounds used in this study.
Table 2. IC50, MIC and MBC values of the tested compounds.
| Source | Name | IC50 a | MICa | MBCa |
| QSI-reference | 4-NPO | 24 | 25 | >100 |
| Chemical library | 14 | >100 (0%)b | >100 | >100 |
| 15 | >100 (19%) | >100 | >100 | |
| 283 | 73 | >100 | >100 | |
| 729 | 75 | >100 | >100 | |
| 937 | >100 (15%) | >100 | >100 | |
| 1099 | 35 | 100 | >100 | |
| 1102 | >100 (0%) | >100 | >100 | |
| 1248 | 63 | >100 | >100 | |
| 1289 | >100 (16%) | >100 | >100 | |
| 1577 | 2,5 | 3 | 25 | |
| 1868 | >100 (34%) | 12,5 | >100 | |
| 1949 | >100 (38%) | >100 | >100 | |
| 3028 | >100 (32%) | >100 | >100 | |
| 3492 | 50 | >100 | >100 | |
| 3499 | >100 (19%) | >100 | >100 | |
| 1248-diastereoisomers | (S,S)-1248 | 90 | 100 | >100 |
| (S,R)-1248 | 32 | 100 | 100 | |
| (R,S)-1248 | >100 (0%) | 100 | 100 | |
| (R,R)-1248 | >100 (10%) | 100 | 100 | |
| Commercial products | Jasmonic acid | 25 | >100 | >100 |
| Estradiol | 75 | >100 | >100 | |
| Estriol | 50 | >100 | >100 |
values are in µg/ml.
In brackets, inhibition (%) at 100 µg/ml.
With the exception of compound 1577, none variation of the cell density (OD600) was observed in the IC50 determination assay. To know more about bactericidal activity of these compounds, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were calculated according to the Andrews' recommendations [18]. The QSI-reference 4-NPO weakly inhibited the growth of A. tumefaciens as the MIC value reached 25 µg/ml (Table 2). All the other tested compounds, with the exception of 1577 and 1868 (MIC at 3 and 12.5 µg/ml respectively), exhibited a MIC value equal to or greater than 100 µg/ml, which was the highest concentration tested for determining IC50 value and impact of the compounds on QS-regulated plasmid transfer in A. tumefaciens. The MBC test confirmed the weak toxicity of the studied QSI because all the MBC values were equal to or higher than 100 µg/ml, with the exception of that of 1577 (MBC at 25 µg/ml).
QSIs modulate QS-regulated Ti-plasmid transfer in A. tumefaciens
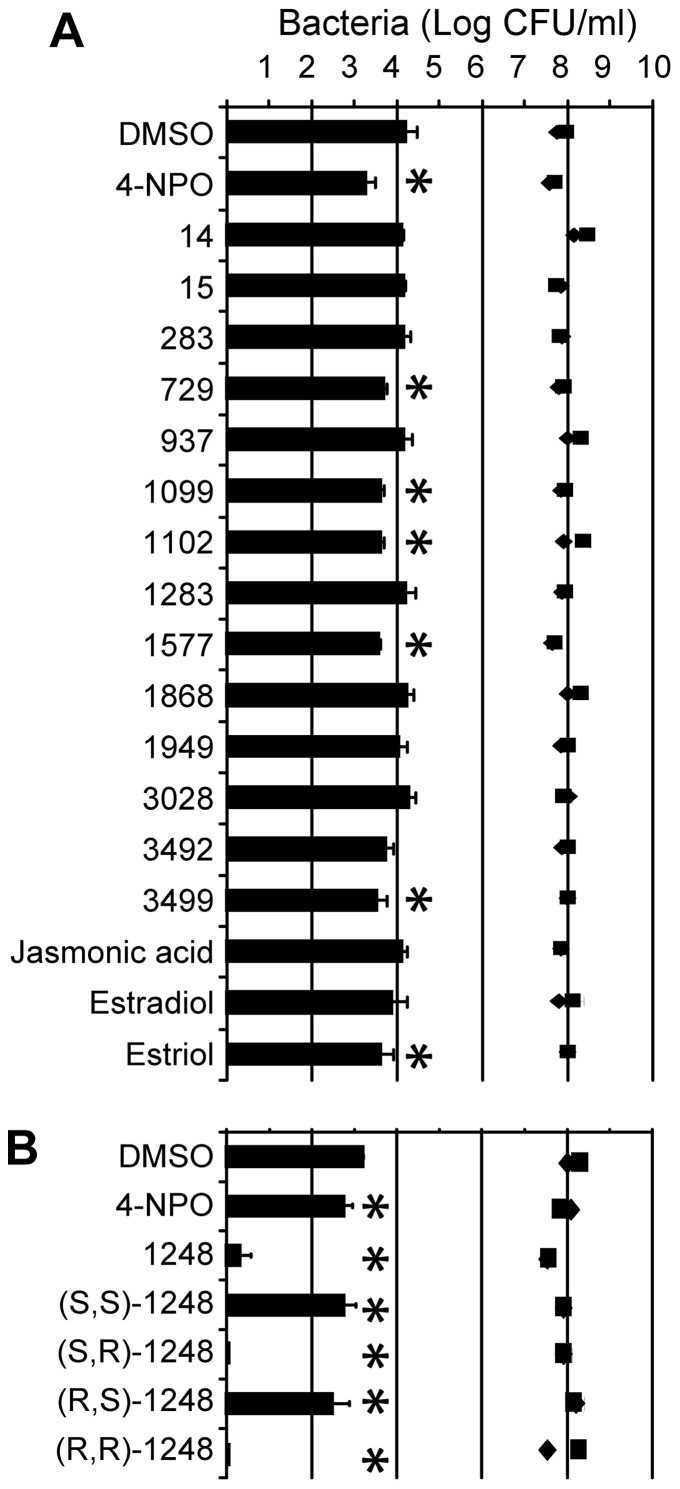
In A. tumefaciens, QS positively regulates horizontal transfer of the virulence plasmid (called Ti plasmid) from a donor strain to a recipient strain. Recipient strains which have received the plasmid are called transconjugants. In this assay, a Ti-plasmid donor and recipient strains were mixed in the presence of AHL (OC8-HSL) and QSI. Resulting transconjugants were counted on rich agar medium supplemented with appropriate antibiotics. When the QSI-reference 4-NPO was added at 0.1 mg/ml, the plasmid transfer efficiency decreased by 10-fold (Figure 4A). Five chemical library-QSIs (729, 1099, 1102, 1577 and 3499) and estriol were also able to significantly reduce the plasmid transfer frequency by one order of magnitude. In another experiment (Figure 4B), the four 1248-diastereoisomers were compared. All of them significantly affected horizontal transfer of the Ti plasmid. Noticeably, in conjugation assays, the level of the donor and recipient cells remained at a high level (around 108 CFU/ml, Figure 4), suggesting that the measured decrease in plasmid transfer would not caused by a toxic effect of the tested compounds.
Figure 4. In vitro Ti plasmid transfer frequency in Agrobacterium.
The Ti plasmid transfer frequencies were measured in the presence of QSI (A) and the four 1248-diastereoisomeres (B) at 0.1 mg/ml. Histograms represent the cell density of transconjugants (CFU/ml), while black diamonds and squares, those of the donor and recipient strains, respectively. Measurements were performed in quadruplicate and the experiment was repeated twice. The cell densities of transconjugants in the presence of QSI were compared to that of the control in the presence of DMSO with a Mann and Whitney test (α = 0.05). Statistically different values are noted by asterisks.
QSIs modulate QS-signal accumulation in P. aeruginosa
Because the assays with the plant pathogen A. tumefaciens highlighted human hormones as QSIs (estrone = 729, estriol, and estradiol), their QSI-activity was evaluated with the opportunistic pathogen P. aeruginosa.
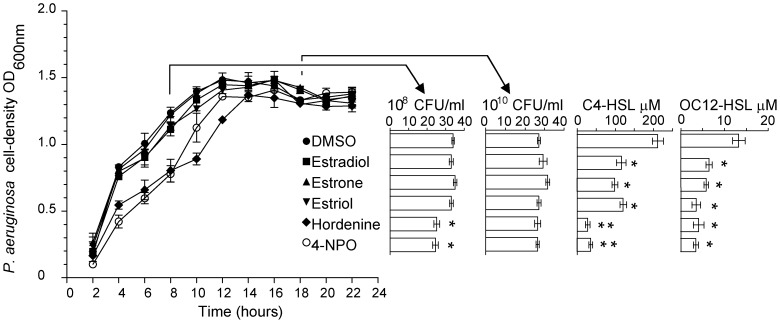
Growth curves of P. aeruginosa cells were determined in the presence of the QSIs (Figure 5). The addition of estradiol, estrone and estriol at 0.5 mg/ml did not affect the growth of P. aeruginosa. Only hordenine and 4-NPO slightly delayed the growth of P. aeruginosa. This was further confirmed by the enumeration of CFU after 8- and 18-hour of incubation. Noticeably, in all cases, the Pseudomonas cells reached the same final cell density at the stationary phase. At 18-hour, the concentrations of the AHLs butyrylhomoserine lactone (C4-HSL) and 3-oxo-dodecanoylhomoserine lactone (OC12-HSL), which are produced by P. aeruginosa, were quantified using mass spectrometry as described by Vandeputte et al. [19]. The presence of the human hormones estradiol, estrone and estriol, as well as that of hordenine and 4-NPO provoked a reduction of the C4-HSL and OC12-HSL concentrations in the cell cultures, suggesting the QS-signal synthesis and QS-signalling were affected.
Figure 5. QSIs modulated QS-signal accumulation in P. aeruginosa.
Growth kinetics (n = 6) of P. aeruginosa was measured (OD600) in the presence of the QSIs (estradiol, estrone, estriol, hordenine and 4-NPO) at 0.5 mg/ml using DMSO as a negative control. Cell counts were assessed (CFU/ml) at 8- and 18-hour, and C4-HSL and OC12-HSL concentration (µM) were determined in the bacterial cultures at 18-hour. Statistically different values (Student's t test with α = 0.01) are noted by asterisks.
QSIs modulate QS-regulated genes in P. aeruginosa
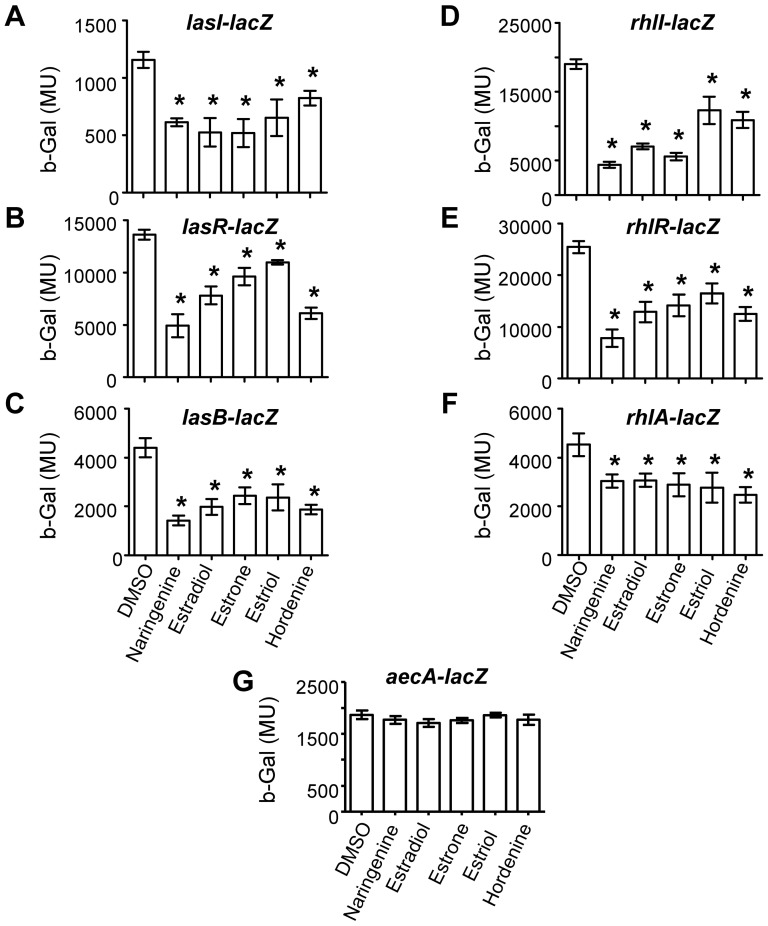
The expression of six QS-regulated genes was measured in P. aeruginosa: the AHL-synthetase genes lasI and rhlI, the AHL-sensor genes lasR and rhlR, and downstream genes lasB and rhlA, which are regulated by the las and rhl systems, respectively (Figure 6). The effect of the QSIs was compared to that of naringenin, a known QSI in P. aeruginosa [19]. All tested compounds markedly affected the expression of the synthetase genes lasI and rhlI, though estriol and hordenine had a lower impact on rhlI expression than naringenin, estradiol and estrone. Expression of the regulators lasR and rhlR was also affected, with hordenine being the most and estriol the least potent inhibitors. Other QS-regulated genes, lasB and rhlA, were also down-regulated in the presence of the tested QSIs. To verify that the drop in β-galactosidase activity of the reporter genes was indeed associated with a reduction in QS-related gene expression and not with a general effect on transcription/translation mechanisms, the activity of the aceA promoter, the expression of which is not regulated by QS [20] was assessed. The addition of the QSIs did not modify the transcription of the aceA gene (Figure 6), indicating that these compounds affected the expression of QS-related genes without affecting the transcription machinery of P. aeruginosa PAO1.
Figure 6. QSIs modulated QS-regulated genes in P. aeruginosa.
The β-galactosidase (b-Gal) activity in Miller unit (MU) of the transcriptional fusions lasI-lacZ (A), lasR-lacZ (B), lasB-lacZ (C), rhlI-lacZ (D), rhlR-lacZ (E), rhlA-lacZ (F), and aecA-lacZ(G) were measured in the presence of estradiol, estrone, estriol, hordenine at 0.5 mg/ml, and naringenin at 1 mg/ml as a positive control and DMSO as a negative control. The statistical significance of each test (n = 5 and three biological replicates) was evaluated by Student's t test (i.e. each test was compared with the DMSO condition). Asterisks indicate statistically different data (p value of ≤0.01).
QSIs modulate activity of the QS-signal sensors LasR and RhlR of P. aeruginosa
We determined whether the AHL-binding transcriptional factors LasR and RhlR were impaired in their capacity to activate expression of the QS-regulated genes in the presence of various QSIs. This was achieved by using two E. coli bioindicator strains expressing either LasR or RhlR proteins and harboring an appropriate reporter lux operon for measuring their transcriptional activity in the presence of OC12-HSL at 100 µM and C4-HSL at 10 µM, respectively [21]. Adding C4-HSL to the pAL101-bioindicator or OC12-HSL to the pAL105-bioindicator induced the expression of the reporter lux operon and the consequent production of luminescence. In contrast, only background levels of luminescence were detected in control strains harboring the plasmids pAL102 or pAL106, which lacks the rhlR or lasR gene, respectively.
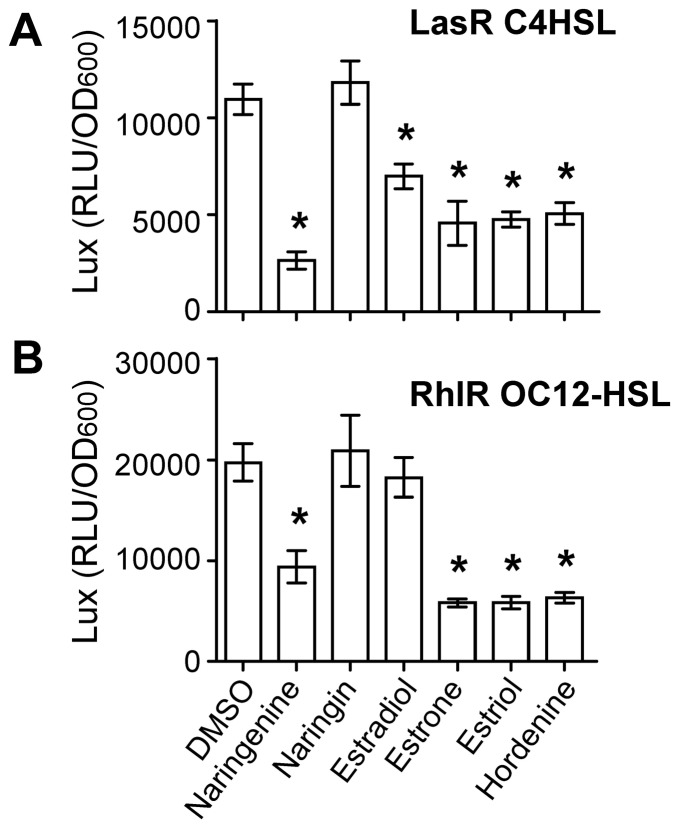
As shown in Figure 7, both biosensor strains produced less luminescence when naringenin was added to the growth medium as compared with DMSO-treated biosensor cells, indicating that the functioning of the LasR and RhlR proteins was impaired, while the structurally-related compound naringin had no effect. Estradiol affected LasR functionality but not that of RhlR. The other QSIs, estrone, estriol and hordenine, had a significant impact on the perception of both OC12-HSL and C4-HSL by LasR and RhlR, respectively. This observation indicated that the inhibition of the expression of the QS genes in P. aeruginosa PAO1 was likely due to a competition between the endogenous AHLs and the tested QSIs to access the AHL-binding site of the LasR and RhlR transcription factors.
Figure 7. QSIs modulated activity of the AHL-sensors in P. aeruginosa.
Luminescence of the reporting operon lux, expressed in relative light units (RLU/OD600), was measured in E. coli bioindicator strains harboring the LasR (A) and RhlR (B) AHL-sensing systems of P. aeruginosa in the presence of C4-HSL and OC12-HSL, respectively. The QSIs estradiol, estrone, estriol and hordenine were added at 0.1 mg/ml, while naringenin and naringin, added at 0.5 mg/ml, were used as QSI-reference and non-QSI reference, respectively. Each test (n = 6) was compared with the DMSO-condition using Student's t test. Asterisks indicate statistically different data (p value of ≤0.01).
Modeling of the QSI-LasR and QSI-TraR interactions
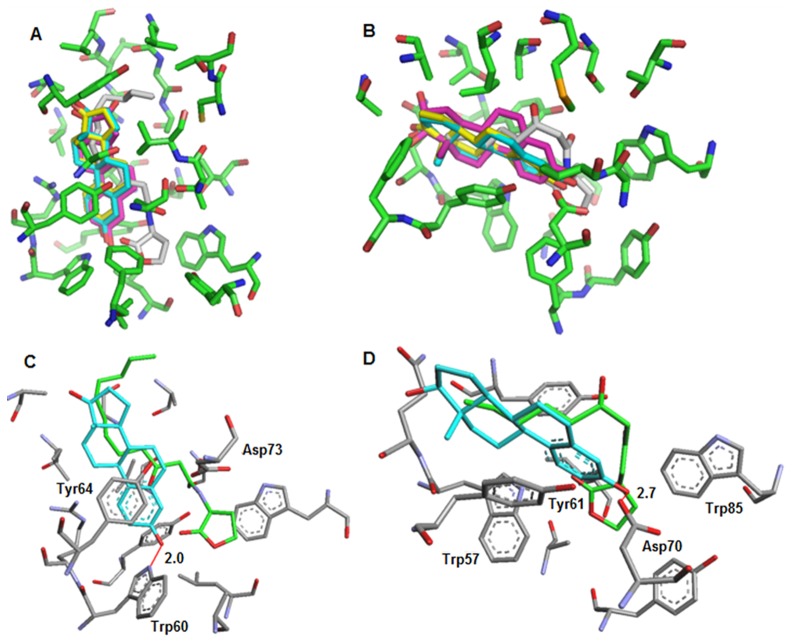
In silico docking experiments of estrone, estriol, and estradiol within the binding sites of LasR and TraR were performed. The structure of RhlR is not available and modeling the docking of QSIs could therefore not be achieved. Superimposition of binding models revealed a similar binding mode for the three hormones within the AHL-binding pocket of either LasR or TraR. Notably, the hormone binding modes depicted in Figure 8 were slightly different for LasR and TraR. Careful examination of these binding modes suggested that the saturated rings of these hormones were located in the protein area interacting with the alkyl chain of QS-signals OC12-HSL and OC8-HSL (Figure 8). In the proposed models, the aromatic ring of hormones interacted with a conserved residue in the LuxR family, namely Tyr64 (LasR) or Tyr61 (TraR), while a hydrogen bond links their phenol group with Trp60 of LasR and Asp70 of TraR. Modeling suggests that the LasR/TraR residues, which were involved in the binding of human hormones, were also required for AHL-binding.
Figure 8. Modeling of interactions between AHL-sensors and human hormones.
Superimposed modeling of the overall binding modes of estradiol (cyan), estriol (magenta), estrone (yellow), or AHLs (OC12-HSL or OC8-HSL in gray) within the binding site of LasR (A) and of TraR (B).Superimposed modeling of the simplified binding modes showing interactions between estradiol (selected as an example in cyan) or natural ligands (OC12-HSL or OC8-HSL in green) and binding sites residues of LasR (C) and TraR (D).
Discussion
This article reports the identification of novel QSIs such as the natural plant compound hordenine and the synthetic indoline-2-carboxamides, and also demonstrates the QSI-activity of the three human sexual hormones that are estrone, estriol and estradiol.
QSIs have been identified in many organisms, plants being the most frequently investigated source of QSI compounds and algae the providers of the most potent ones [1], [5]. Our results revealed the QSI potentiality of alkaloids such as hordenine (ID 283), 1248, and 3492. Hordenin (CAS# 3595-05-9) is a natural alkaloid of the phenethylamine class exhibiting a widespread occurrence in plants (ornamentals, fruits and vegetables), including those that are used for human and animal consumption [22]–[23]. Following injection, hordenine stimulates the release of norepinephrine in mammals hence acting indirectly as an adrenergic drug [24]–[25]. In the literature, alkaloid compounds have been less frequently reported as acting as QSI than aromatic or polyaromatic compounds [1]. Indeed, solenopsin A, a venom alkaloid produced by the fire ant Solenopsis invicta, has been shown to inhibit biofilm formation, pyocyanin and elastase production as well as the expression of QS-regulated genes lasB, rhlI and lasI in P. aeruginosa [26]. Peters and co-workers [27] also demonstrated that brominated tryptamine-based alkaloids from Flustrafoliacea, a sea bryozoan, inhibit AHL-regulated gene expression using biosensors P. putida (pKR-C12), P. putida (pAS-C8) and E. coli (pSB403) lasR, cepR and luxR coupled to the promoter of lasB, cepI and luxI, respectively.
In this study, the QSI activity of human hormones was supported by complementary features. The pure hormones, especially estriol and estrone, affected expression of the QS-regulated reporter fusion traG-lacZ and QS-dependent horizontal transfer of the virulence Ti-plasmid in A. tumefaciens. They also decreased the expression of six QS-regulated genes lasI, lasR, lasB, rhlI, rhlR, and rhlA in P. aeruginosa, but none decreased expression of the QS-independent gene aceA. Because of the effect on lasI and rhlI, the AHL concentration was also affected in the presence of the sexual hormones. In agreement with a previous report comparing the effect of steroid hormones on the growth of several pathogens [28], they did not affect the growth of A. tumefaciens and P. aeruginosa at the concentrations used for describing QSI activity. The sexual hormones act as QSIs at a mM-range concentration which is similar to that of the natural polycyclic QSIs such as catechin and naringenin [19], [29], but higher than that of some other natural and synthetic QSIs which act at a µM range or lower [3], [30]. Our work also revealed that pure hormones affected the QS-regulated reporter gene of P. aeruginosa when RhlR or LasR was expressed in E. coli in the presence of the appropriate AHL. Moreover, molecular modeling confirmed the competitive hormone-binding capacity of the two AHL-sensors LasR and TraR, suggesting that the AHL-LuxR sensors are targets of the discovered QSIs. This mechanism of action is frequently encountered among QSIs [3]. Such a putative cross-talk between QS and hormonal signalling was hypothesized in prospective reviews by Rumbaugh [31] and Hughes and Sperandio [32] and in a paper reporting docking-type screening of QSIs [33], but, to our knowledge, was never experimentally observed in vitro until this report.
Finally, the hypothesis rose about QSI-activity of sexual hormones in vivo because the opportunistic pathogen P. aeruginosa is detectable in several tissues and organs of hospitalized patients and healthy women, and can thus come into contact with sexual hormones [34]–[36]. A major argument against this hypothesis is that QSI activity of hormones was observed at 2 mM (0.5 mg/ml) while, in serum, concentrations of hormones such as estradiol reach up to 0.4–1.6 nM (100–400 ng/ml) in healthy women and 2–18 nM during fertilizing protocols [37]. However, the debate remains still unclosed because clinical and environmental Pseudomonas isolates are known for their capacity to import, bind and biodegrade human hormones, including estrogens, via proteins and pathways that are still poorly-characterized [38]–[40]. These hormone-modifying capabilities would contribute to underestimate the QSI-efficiency of hormones in our in vitro assay.
Materials and Methods
Instrumentation
Infrared spectra were recorded on a Perkin Elmer Spectrum BX FT-IR spectrometer. Proton (1H) and carbon (13C) NMR spectra were recorded on Bruker spectrometers: Avance 300 MHz (QNP - 13C- probe or Dual 13C probe) and Avance 500 MHz (BB0 - ATM probe or BBI - ATM probe). Carbon NMR (13C) spectra were recorded at 125 or 75 MHz, using a broadband decoupled mode with the multiplicities obtained using a JMOD or DEPT sequence. NMR experiments were carried out in deuterochloroform (CDCl3) or dimethyl sulfoxide (d6-DMSO), chemical shifts (δ) are reported in parts per million (ppm) with reference to CDCl3 (1H: 7.24; 13C: 77.23). The following abbreviations are used for the proton spectra multiplicities: s: singlet, bs: broad singlet, d: doublet, t: triplet, q: quartet, hept: heptuplet, m: multiplet, br: broad. Coupling constants (J) are reported in Hertz (Hz). Mass spectra were obtained either with a LCT (Micromass) instrument using electrospray ionization (ES), or from a Time of Flight analyzer (ESI-MS) for the high resolution mass spectra (HRMS). The purity and the exact mass were determined for ID 1949 and ID 283 with a Waters Acquity liquid chromatograph equipped with a Photodiode Array Detector, an Evaporative Light Scattering Detector and a Triple Quadripole Detector. A reverse-phase HSS T3 column, 2.6 µm, 4.6×100 mm was used for the UPLC work with a mixture acetonitrile/water as the solvent system.
Elemental analyses were performed on a Perkin Elmer CHN 2400 analyzer with detection by catharometry. Thin-layer chromatography was performed on silica gel 60 F254 on aluminium plates (Merck) and visualized under a UVP Mineralight UVLS-28 lamp (254 nm) and with ninhydrin and p-anisaldehyde in ethanol. Flash chromatography was conducted on Merck silica gel 60 (40–63 µm) at medium pressure (300 mbar) or on CombiFlash apparatus (Serlabo Technologies), using standard settings. Reagents and substrates were purchased from Sigma-Aldrich Chemical Company.
Compounds: General Procedures
Procedure A-Preparation of carboxamides IIIa-d: To a solution of the indolinylcarboxylic acid I (1 eq) in dry methylene chloride (0.3M) at 0°C, was added the amine II (1.05 eq), 1-hydroxybenzotriazole (HOBt, 1.05 eq), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI, 1.05 eq) and triethylamine (1.05 eq). The mixture was stirred at 0°C for 1 h and at room temperature for 5 h. The reaction mixture was quenched with water and extracted with methylene chloride. The organic phase was washed successively with saturated aqueous Na2SO4 and saturated aqueous NaCl, dried over MgSO4, filtered and concentrated under reduce pressure. The residue was purified by flash chromatography on silica gel (elution with heptane/EtOAc 0 to 20%).
Procedure B-Reduction of carboxamides IIIa-d: To a solution of the amide derivatives IIIa-d (1 eq) in dry THF (0.03M) at 0°C, was added BH3.THF (1M, 1 eq) and the mixture was stirred at reflux for 16 h. The reaction mixture was cooled to 0°C, acidified with aqueous HCl (2M) and refluxed for an additional 30 min. The mixture was then extracted with methylene chloride and the organic phase was washed successively with saturated aqueous Na2CO3, saturated aqueous NaCl, dried over MgSO4, filtered and concentrated under reduce pressure. The residue was purified by flash chromatography on silica gel (elution with heptane/EtOAc 0 to 30%). The pure amine product was dissolved in diethyl ether, a solution of HCl in diethyl ether (2M) was added and the precipitated hydrochloride salt was collected by filtration, washed with diethyl ether and dried under vacuum.
(S)-N-((S)-1-(naphthalen-1-yl)ethyl)indoline-2-carboxamide (IIIa)
Following general procedure A using (S)-indoline-2-carboxylic acid (0.61 mmol, 100 mg), (S)-1-(1-naphthyl)ethylamine (0.64 mmol, 103 µL), HOBt (0.64 mmol, 86 mg), EDCl (0.64 mmol, 123 mg) and Et3N (0.64 mmol, 46 µL) in methylenechloride (2 mL), IIIa was obtained as a white solid (182 mg, 94%). 1H NMR (CDCl3): 8.17 (d, J = 8.3 Hz, 1H), 7.91 (d, J = 7.3 Hz, 1H), 7.84 (d, J = 8.3 Hz, 1H), 7.60−7.46 (m, 4H), 7.36−7.34 (m, 1H), 7.14 (d, J = 7.3 Hz, 1H), 7.07 (t, J = 8.3 Hz, 1H), 6.84 (td, J = 7.3, 1.0 Hz, 1H), 6.65 (d, J = 7.3 Hz, 1H), 6.04−5.95 (m, 1H), 4.46−4.40 (m, 1H), 4.05 (bs, 1H), 3.69−3.60 (m, 1H), 3.19−3.11 (m, 1H), 1.68 (d, J = 7.0 Hz, 3H); 13C NMR (CDCl3): 172.6, 149.5, 138.2, 134.0, 131.2, 128.8, 128.4, 128.0, 127.6, 125.9, 125.3, 124.8, 124.3, 123.4, 122.7, 120.6, 110.8, 61.4, 44.0, 35.6, 20.7; IR (neat) 3298, 2900, 1651, 1608, 1512, 1484, 1467, 1245; MS (ESI): [M+H] m/z 317.2.
(S)-N-((R)-1-(naphthalen-1-yl)ethyl)indoline-2-carboxamide (IIIb)
Following general procedure A using (S)-indoline-2-carboxylic acid (0.61 mmol, 100 mg), (R)-1-(1-naphthyl)ethylamine (0.64 mmol, 103 µL), HOBt (0.64 mmol, 86 mg), EDCl (0.64 mmol, 123 mg) and Et3N (0. mmol, 46 µL) in methylenechloride (2 mL), IIIb was obtained as a white solid (105 mg, 54%). 1H NMR (CDCl3): 8.12 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.56−7.40 (m, 4H), 7.36−7.31 (m, 1H), 7.04 (t, J = 7.6 Hz, 2H), 6.78 (td, J = 7.3, 1.2 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 6.05−5.95 (m, 1H), 4.53−4.47 (m, 1H), 4.15 (bs, 1H), 3.60−3.49 (m, 1H), 2.99−2.91 (m, 1H), 1.72 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3): 172.4, 149.5, 138.3, 133.9, 131.1, 128.8, 128.3, 127.9, 127.6, 126.4, 125.8, 125.2, 124.8, 123.3, 122.3, 120.6, 110.8, 62.0, 44.1, 35.5, 21.2; IR (neat) 3298, 2900, 1651, 1608, 1512, 1484, 1467, 1245; MS (ESI): [M+H] m/z 317.2.
(R)-N-((S)-1-(naphthalen-1-yl)ethyl)indoline-2-carboxamide (IIIc)
Following general procedure A using (R)-indoline-2-carboxylic acid (0.61 mmol, 100 mg), (S)-1-(1-naphthyl)ethylamine (0.64 mmol, 103 µL), HOBt (0.64 mmol, 86 mg), EDCl (0.64 mmol, 123 mg) and Et3N (0.64 mmol, 46 µL) in methylenechloride (2 mL), IIIc was obtained as a white solid (141 mg, 73%). 1H NMR (CDCl3): 8.12 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.80 (d, J = 8.3 Hz, 1H), 7.56−7.40 (m, 4H), 7.36−7.31 (m, 1H), 7.04 (t, J = 7.6 Hz, 2H), 6.79 (td, J = 7.3, 1.2 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 6.05−5.95 (m, 1H), 4.50−4.48 (m, 1H), 4.15 (bs, 1H), 3.61−3.49 (m, 1H), 3.00−2.87 (m, 1H), 1.72 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3): 172.4, 149.5, 138.3, 133.9, 131.1, 128.8, 128.3, 127.9, 127.6, 126.4, 125.8, 125.2, 124.8, 123.3, 122.3, 120.6, 110.8, 62.0, 44.1, 35.5, 21.2; IR (neat) 3352, 2900, 1651, 1608, 1511, 1484, 1466, 1244; MS (ESI): [M+H] m/z 317.2.
(R)-N-((R)-1-(naphthalen-1-yl)ethyl)indoline-2-carboxamide (IIId)
Following general procedure A using (R)-indoline-2-carboxylic acid (0.61 mmol, 100 mg), (R)-(1-(1-naphthyl)ethylamine (0.64 mmol, 103 µL), HOBt (0.64 mmol, 86 mg), EDCl (0.64 mmol, 123 mg) and Et3N (0.64 mmol, 46 µL) in methylenechloride (2 mL), IIId was obtained as a white solid (116 mg, 60%). 1H NMR (CDCl3): 8.12 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.80 (d, J = 8.3 Hz, 1H), 7.56−7.40 (m, 4H), 7.36−7.31 (m, 1H), 7.04 (t, J = 7.6 Hz, 2H), 6.79 (td, J = 7.3, 1.2 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 6.05−5.95 (m, 1H), 4.50−4.48 (m, 1H), 4.01 (bs, 1H), 3.61−3.49 (m, 1H), 3.00−2.87 (m, 1H), 1.68 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3): 172.6, 149.5, 138.3, 133.9, 131.1, 128.8, 128.3, 127.9, 127.6, 126.4, 125.8, 125.2, 124.8, 123.3, 122.3, 120.6, 110.8, 61.4, 44.0, 35.6, 20.7; IR (neat) 3352, 2925, 2900, 1651, 1608, 1511, 1484, 1466, 1246; MS (ESI): [M+H] m/z 317.2.
(S)-N-((S)-indolin-2-ylmethyl)-1-(naphthalen-1-yl)ethanamine ((S,S)-1248)
Following the general procedure B using amide IIIa (0.25 mmol, 80 mg), BH3.THF (1M, 1.21 mL) in dry THF (8 mL) afforded ((S,S)-1248 (42 mg, 55%) as a colorless oil. 1H NMR (CDCl3): 8.19 (dd, J = 7.1, 2.1 Hz, 1H), 7.87 (dd, J = 7.1, 2.1 Hz, 1H), 7.76 (d, J = 8.1 Hz, 1H), 7.64 (d, J = 8.1 Hz, 1H), 7.54−7.45 (m, 3H), 7.06−6.98 (m, 2H), 6.66 (t, J = 7.1 Hz, 1H), 6.62 (d, J = 8.1 Hz, 1H), 4.75−4.60 (m, 1H), 3.94−3.84 (m, 1H), 3.09 (dd, J = 15.9, 8.9 Hz, 1H), 2.72−2.65 (m, 3H), 1.51 (d, J = 6.7 Hz, 3H); 13C NMR (CDCl3): 150.8, 141.3, 134.0, 131.3, 129.0, 128.7, 127.3, 127.2, 125.9, 125.7, 125.4, 124.8, 123.0, 122.7, 118.7, 109.7, 59.3, 54.0, 53.3, 34.1, 23.6; IR (neat) 3361, 2973, 2926, 2853, 1609, 1485, 1465, 1115; HRMS (ESI): calcd. for C21H23N2 [M+H] m/z 303.1861, found m/z 303.1863. Hydrochloride derivative: Anal. Calcd. C, 67.20; H, 6.45; N, 7.46. Found C, 62.07; H, 6.22; N, 6.76.
(R)-N-((S)-indolin-2-ylmethyl)-1-(naphthalen-1-yl)ethanamine ((S,R)-1248)
Following general procedure B using amide IIIb (0.30 mmol, 95 mg), BH3.THF (1M, 1.45 mL) in dry THF afforded ((R,S)-1248 (74 mg, 81%) as a colorless oil. 1H NMR (CDCl3): 8.31 (d, J = 8.5 Hz, 1H), 7.97 (d, J = 7.2 Hz, 1H), 7.83 (d, J = 8.5 Hz, 1H), 7.74 (d, J = 7.2 Hz, 1H), 7.64−7.53 (m, 3H), 7.15−7.08 (m, 2H), 6.78 (t, J = 7.2 Hz, 1H), 6.68 (d, J = 7.8 Hz, 1H), 4.77−4.70 (m, 1H), 4.04−3.92 (m, 1H), 3.20−3.12 (m, 1H), 2.86−2.78 (m, 2H), 2.72−2.66 (m, 1H), 1.62 (d, J = 6.5 Hz, 3H); 13C NMR (CDCl3): 150.9, 140.9, 134.1, 131.4, 129.1, 128.7, 127.3, 127.0, 125.8, 125.8, 125.4, 124.9, 123.1, 122.9, 118.7, 109.7, 59.1, 53.9, 52.8, 33.9, 23.8; IR (neat) 3363, 2973, 2927, 2864, 1608, 1484, 1464, 1247, 1114; HRMS (ESI): calcd. for C21H23N2 [M+H] m/z 303.1861, found m/z 303.1873. Hydrochloride derivative: Anal. Calcd. C, 67.20; H, 6.45; N, 7.46. Found C, 67.27; H, 6.78; N, 7.25.
(S)-N-((R)-indolin-2-ylmethyl)-1-(naphthalen-1-yl)ethanamine ((R,S)-1248)
Following general procedure B using amide IIIc (0.35 mmol, 110 mg), BH3.THF (1M, 1.70 mL) in dry THF (10 mL) afforded (S,R)-1248 (76 mg, 72%) as a colorless oil. 1H NMR (CDCl3): 8.31 (d, J = 8.5 Hz, 1H), 7.96 (d, J = 7.2 Hz, 1H), 7.83 (d, J = 8.5 Hz, 1H), 7.74 (d, J = 7.2 Hz, 1H), 7.64−7.53 (m, 3H), 7.15−7.08 (m, 2H), 6.78 (t, J = 7.2 Hz, 1H), 6.68 (d, J = 7.8 Hz, 1H), 4.77−4.70 (m, 1H), 4.04−3.92 (m, 1H), 3.20−3.12 (m, 1H), 2.86−2.78 (m, 2H), 2.72−2.66 (m, 1H), 1.62 (d, J = 6.5 Hz, 3H); 13C NMR (CDCl3): 150.9, 141.0, 134.1, 131.4, 129.1, 128.7, 127.3, 127.0, 125.8, 125.8, 125.4, 124.9, 123.1, 122.9, 118.7, 109.7, 59.1, 53.9, 52.8, 33.9, 23.8; IR (neat) 3364, 2973, 2927, 2863, 1609, 1484, 1464, 1247, 1114; HRMS (ESI): calcd. for C21H23N2 [M+H] m/z 303.1861, found m/z 303.1862. Hydrochloride derivative: Anal. Calcd. C, 67.20; H, 6.45; N, 7.46. Found C, 68.78; H, 6.78; N, 7.20.
(R)-N-((R)-indolin-2-ylmethyl)-1-(naphthalen-1-yl)ethanamine ((R,R)-1248)
Following general procedure B using amide IIId (0.35 mmol, 110 mg), BH3.THF (1M, 1.70 mL) in dry THF (10 mL) afforded (R,R)-1248 (76 mg, 72%) as a colorless oil. 1H NMR (CDCl3): 8.27 (d, J = 8.3 Hz, 1H), 7.96 (d, J = 7.1 Hz, 1H), 7.83 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 7.1 Hz, 1H), 7.62−7.53 (m, 3H), 7.14−7.07 (m, 2H), 6.77 (t, J = 7.1 Hz, 1H), 6.69 (d, J = 7.1 Hz, 1H), 4.71 (q, J = 7.1 Hz, 1H), 4.00−3.90 (m, 1H), 3.20−3.12 (m, 1H), 2.79−2.72 (m, 3H), 1.59 (d, J = 6.7 Hz, 3H); 13C NMR (CDCl3): 150.8, 141.4, 134.1, 131.3, 129.1, 128.8, 127.3, 127.0, 125.9, 125.7, 125.4, 124.9, 123.1, 122.9, 118.7, 109.8, 59.3, 54.0, 53.4, 34.1, 23.7; IR (neat) 3364, 2973, 2927, 2863, 1609, 1484, 1464, 1247, 1114; HRMS (ESI): calcd. for C21H23N2 [M+H] m/z 303.1861, found m/z 303.1867. Hydrochloride derivative: Anal. Calcd. C, 67.20; H, 6.45; N, 7.46. Found C, 67.64; H, 6.91; N, 7.10.
2,3,11a-Trimethyl-2,3,3a,4,5,5a,5b,6,8,9,10,11,11a,11b,12,13-hexadecahydro-1H-2-aza-pentaleno[1,6a-a]phenanthren-9-ylamine hydrochloride or dihydroconaminehydrochloride ID 283
1H NMR (d6-DMSO): 0.65-0.70 (1H, m, H9), 0.72 (3H, s, H20), 0.97–1.00 (3H, m, H1a H4b H12b), 1.11–1.19 (4H, m, H8 H10 H14), 1.20–1.24 (1H, m, H3a), 1.28 (3H, d, J = 6.3 Hz, H22), 1.34–1.45 (4H, m, H7 H11a H15a), 1.50–1.54 (1H, m, H16b), 1.57–1.68 (3H, m, H4b H11b H12b), 1.70–1.76 (1H, m, H15b), 1.81–1.87 (1H, m, H16a), 1.99–2.03 (1H, m, H3b), 2.16–2.22 (2H, m, H4), 2.69 (3H, s, H21), 2.86–3.01 (1H, m, H2), 3.35–3.40 (2H, m, H19), 3.45–3.59 (1H, m, H18), 8.10 (2H, s, NH2). 13C NMR(d6-DMSO): 11.2 (1C, C22), 11.7 (1C, C20), 21.3 (1C, C12), 21.7 (1C, C16),25.4 (1C, C11), 25.7 (1C, C15), 27.7 (1C, C8), 31.4 (1C, C4), 32.1 (1C, C7), 35.0 (1C, C5), 35.9 (2C, C1 C3),36.9 (1C, C14),39.3 (1C, C31),43.8 (1C, C10), 49.3 (1C, C2), 51.3 (1C, C13),51.7 (1C, C17), 52.3 (1C, C9), 53.8 (1C, C6), 59.6 (1C, C19), 64.6 (1C, C18). MS (ESI, m/z)331.3 [M+H]+.
3-Hydroxy-13-methyl-6,7,8,9,11,12,13,14,15,17-decahydro-cyclopenta[a] phenanthren-16-one ID 729
1H NMR (d6-DMSO): 0.84 (3H, s, H18), 1.30–1.45 (3H, m, H8a H9 H11a), 1.58 (1H, dt, J1 = 3.3 Hz, J2 = 12.3 Hz, H12a), 1.72–1.79 (2H, m, H8b H14), 1.85 (1H, dt, J1 = 3.3 Hz, J2 = 12.3 Hz, H12b), 1.93–1.99 (1H, m, H15a), 2.03–2.06(1H, m, H17), 2.12–2.16 (1H, m, H15b), 2.27–2.33 (2H, m, H10 H11b), 2.71–2.76 (2H, m, H7), 6.42 (1H, d, J = 2.4 Hz, H1), 6.51 (1H, dd, J1 = 2.4 Hz, J2 = 8.4 Hz, H3), 7.4 (1H, d, J = 8.7 Hz, H4), 8.99 (1H, s, OH). 13C NMR(d6-DMSO):17.8 (1C, C18), 25.8, (1C, C7), 27.6 (1C, C8), 29.0 (1C, C11), 37.3 (1C, C12), 37.7 (1C, C14), 38.3 (1C, C15), 38.8 (1C, C13), 43.1 (1C, C10), 49.7 (1C, C9), 55.3 (1C, C17), 112.7, (1C, C3),114.91 (1C, C1), 125.77 (1C, C4), 130.11 (1C, C5), 136.9 (1C, C6), 154.9 (1C, C2), 217.1 (1C, C16). MS (IE (70 eV), m/z)341.2 [M]+.
5-Methyl-1-(2-phenylsulfanyl-ethoxymethyl)-1H-pyrimidine-2,4-dione ID 1949
1H NMR (d6-DMSO), 1.75 (3H, s, H18), 3.13 (2H, t, J = 6 Hz, H10), 3.64 (2H, t, J = 6 Hz, H9), 5.05 (2H, s, H7), 7.16–7.733 (5H, m, Ar), 7.54 (1H, s, H6),11.29 (1H, s, NH). 13C NMR(d6-DMSO): 11.8 (1C, C18), 31.8 (1C, C10), 66.83 (1C, C9), 75.9 (1C, C7), 110.00 (1C, C5), 125.8 (1C, C15), 128.2 (2C, C14 C16), 128.9 (2C, C13 C17), 136.0 (1C, C12), 140.3 (1C, C6), 152.0 (1C, C2), 166.0 (1C, C4). MS(ESI, m/z)393.9 [M+H]+.
QS-bioindicators
Two bacterial QS-signal biosensors were used. Chromobacterium violaceum CV026 [41] was grown in Luria-Bertani modified (LBm) medium, in which the NaCl concentration was 5 g/l instead of 10 g/l. Agrobacterium tumefaciens NT1(pZLR4) [42] was grown in AB minimal medium [43] supplemented with mannitol (0.2%). After supplementation with appropriate AHL, each of the QS-biosensors expresses a reporting activity, which is the production of the pigment violacein in C. violaceum CV026 and that of β-galactosidase from the transcriptional fusion traG::lacZ in A. tumefaciens NT1(pZLR4).
Library screening for QSI
When the screening was performed, the chemical library of the Institut de Chimie des Substances Naturelles (ICSN, Gif-sur-Yvette, France) contained more than 3500 synthetic and natural compounds, which were individually dissolved in dimethylsulfoxide (DMSO) at 1mg/ml and stored in 96 microwell plates. A first screening was performed by mixing biosensor CV026 with the AHL hexanoylhomoserine lactone (C6-HSL) at 0.5 µM and the tested compounds at 50 µg/ml. Final volume was adjusted to 100 µl with a synthetic medium (10% LBm and 0.4% sucrose). After 24 hours of incubation at 30°C, the presence or absence of violacein pigment was quoted by visual reading. A second screening was performed using biosensor A. tumefaciens NT1(pZLR4), the AHL octanoylhomoserine lactone (C8-HSL) at 10 nM and the tested molecules at 5 µg/ml. Final volume was adjusted to 100 µl by the addition of AB minimal medium supplemented with mannitol (0.2%) and TY medium (10%). After 4 hours of incubation at 30°C, β-galactosidase activity was measured in A. tumefaciens cultures as previously described [44]. In the two screenings, 4-nitropyridine-N-oxide (4-NPO) was used as a QSI reference [17].
Measurement of IC50, MIC and MBC values of the QSI
To determine half maximal inhibitory concentration (IC50) of QSI, β-galactosidase activity of the Agrobacterium biosensor was measured, as described in the screening protocol, in the presence of AHL and QSI, which were introduced at final concentrations ranging from 1.5 µg/ml to 100 µg/ml. Aside from the effect of QSI on QS regulation, the toxicity of these compounds was tested on bacterial cells by measuring two parameters [18]: the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). MIC, which is the lowest concentration of QSI (µg/ml) inhibiting any visible culture after 36 h of incubation at 30°C, was estimated by culturing 105 CFU/ml of the Agrobacterium biosensor cells in the presence of different concentrations of the QSI. MBC, which is the lowest concentration of QSI (µg/ml) that results in a 99.9% reduction of the initial bacterial population (105 CFU/ml) after 36 h of incubation at 30°C in the presence of different concentrations of QSI, was estimated by plating 100 µL of the Agrobacterium cultures on agar LBm plates. After an incubation of 48 h at 30°C, CFU were counted and the MBC values were calculated.
QS-regulated plasmid-transfer in A. tumefaciens
In A. tumefaciens, the transfer of the Ti-plasmid from a donor cell to a recipient one is controlled by QS. QSI were evaluated for their capacity to reduce plasmid transfer frequency in Agrobacterium. In the plasmid transfer assay, two modified Agrobacterium strains were used: a Ti-plasmid donor, which requires exogenous AHL to transfer a kanamycin-resistant (Kmr) Ti-plasmid [45], and a rifampicin-resistant (Rifr) recipient strain, which is free of Ti-plasmid. Overnight cultures of the donor and recipient strains were mixed with 500 pM of oxo-octanoylhomoserine lactone (OC8-HSL) and 0.1 mg/ml of QSI in LBm medium. Each combination was repeated 4 times in microwell plates. After 72 hours of incubation at 24°C, the recipient and donor cells, as well as recipient cell which had acquired the Ti-plasmid from the donors, were counted on LBm agar plates supplemented with the antibiotics Km and Rif.
QS-regulated genes in Pseudomonas aeruginosa and AHLs quantification
QS gene transcription in P. aeruginosa PAO1 was monitored using PAO1 strains carrying various gene promoters fused to a lacZ reporter gene, as described in Vandeputte et al., 2010 and Table 1. PAO1 reporter strains were prepared according to Vandeputte et al. [29]. Briefly, 18-hours-old liquid cultures (50 µl) were diluted in order to obtain a starting OD600 nm comprised between 0.02 and 0.03 in fresh LB medium supplemented with 50 mM MOPS pH 7.0 (1 ml), carbenicillin (300 µg/ml) and 10 µl of the QSIs to be tested (OD were measured using a SpectraMax M2 device from Molecular Devices). Test and control QSIs were diluted in 100% DMSO (resulting after addition to the growth medium in a 1% final concentration of DMSO). Cultures were incubated for 18 hours at 37°C with agitation. After incubation, cell densities were assessed spectrophotometrically (OD600 nm) and β-galactosidase assays were performed using the substrate o-nitrophenyl-β-D-galactopyranoside as described previously [19], [29]. Promoterless-lacZ fusions were used as controls. AHLs were quantified as described in Vandeputte et al. [19]. All tests were performed in quintuplicates and three biological repetitions. The statistical significance of each test was evaluated by conducting Student's t tests and two-way ANOVA combined with the Tukey post-analysis test using the GraphPad Prism software and p values≤0.01 were considered significant.
Heterologous expression of the Pseudomonas QS-receptors in E. coli
Escherichia coli JLD271 biosensor strains harboring LasR- and RhlR-based plasmids pAL105 and pAL101 and control plasmids pAL106 (LasR-) and pAL102 (RhlR−) (Table 1) were prepared according to Vandeputte et al. [19]. Briefly, these strains were grown in LB medium supplemented with tetracycline (10 µg/ml) and chloramphenicol (25 µg/ml) for 24 h [21]. Then 50-µl portions of the cultures were subcultured in 1 ml of LB medium (the starting OD600 ranged between 0.02 and 0.025 corresponding to 5.106 CFU) supplemented with 10 µl of DMSO (1% [vol/vol] final), 10 µl of naringenin or naringin dissolved in DMSO (2 mM, final concentration), or 10 µl of the molecule to be tested. To induce the expression of the lux operon, 0, 1, 10 or 100 mM of C4-HSL was added to pAL101 and pAL102, while OC12-HSL was added to pAL105 and pAL106. After incubation for 2 h at 37°C with agitation (175 rpm), 200 µl of culture was transferred to 96-well OptiPlate-96 F plates from Perkin-Elmer, and the luminescence of each sample was measured by using a TopCount NXT device from Perkin-Elmer. The LasR(pAL106) and RhlR(pAL102) biosensors were used for background subtraction, and the OD600 values were measured to account for the differences in cell density. All experiments were performed in six replicates. The statistical significance of each test was evaluated by conducting Student's t tests using the GraphPad Prism software, and p values ≤0.05 were considered significant. Naringenin (4′,5,7-trihydroxyflavanone), naringin (4′,5,7-trihydroxyflavanone 7-rhamnoglucoside), the AHLs OC12-HSL and C4-HSL were purchased from Sigma-Aldrich and dissolved freshly in 100% DMSO before use. Tested QSIs were dissolved in 100% DMSO before use.
Modeling of the interactions between QSIs and QS-receptors
PDB codes of the estradiol, estriol and estrone were respectively 2YJA (name EST), 1×8 V (name ESL) and 3HM1 (name J3Z) [46]–[47]. Docking experiments were performed on each hormone using Arguslab [48] with X-ray protein structures of LasR (pdb code 2UV0) [49] and TraR (pdb code1L3L) [50]. The docking engine GA (genetic algorithm) was employed with default parameters using a 15 Å docking box centered on natural ligands (OC12-HSL and OC8-HSL). Docking results for each protein were then superimposed with PyMol(http://www.pymol.org/) to generate figures presented in Figure 8 A and B (top). Simplified binding modes (Figure 8 C and D) were constructed with Arguslab using the docking results of estradiol (taken as example) within LasR and TraR by hiding some binding site residues. Hydrogen bonds were assigned within a distance of 3.0 Å.
Acknowledgments
We would like to thank Pr. Farrand (University of Illinois, USA) for kindly providing the pTi-conjugative strain, Pr. Iglewski (Rochester University, USA) for plasmids pPCS223, pPCS1001, pLPR1 and pPCS1002, Pr J. Kato (Hiroshima University, Japan) for plasmids pQF50, pβ01 and pβ02, Pr Ahmer (Ohio State University, USA) for the pAL-biosensors, and Pr Görisch (Technische Universität, Berlin, Germany) for plasmid pTB4124. We also thank O. Pamlard and S. Beaupierre (ICSN, CNRS, France) for technical assistance.
Funding Statement
OMV was supported by FRS-FNRS (Belgium). TR is indebted to the AUF (Agence Universitaire de la Francophonie, Madagascar – Belgium) and the CUD (Coopération Universitaire pour le Développement, Belgium). The authors thank CNRS (France) and ANR (project SVSE7 2011 ECORUM) for financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR (2011) Applications of small molecule activators and inhibitors of quorum-sensing in gram-negative bacteria. Chem Rev 111: 28–67. [DOI] [PubMed] [Google Scholar]
- 2. Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25: 365–404. [DOI] [PubMed] [Google Scholar]
- 3. Janssens JC, De Keersmaecker SC, De Vos DE, Vanderleyden J (2008) Small molecules for interference with cell-cell-communication systems in Gram-negative bacteria. Curr Med Chem 15: 2144–2156. [DOI] [PubMed] [Google Scholar]
- 4. Amara N, Krom BP, Kaufmann GF, Meijler MM (2011) Macromolecular inhibition of quorum sensing: enzymes, antibodies, and beyond. Chem Rev 111: 195–208. [DOI] [PubMed] [Google Scholar]
- 5. Kalia VC (2013) Quorum sensing inhibitors: An overview. Biotechnol Adv 31: 224–245. [DOI] [PubMed] [Google Scholar]
- 6. Brackman G, Cos P, Maes L, Nelis HJ, Coenye T (2011) Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother 55: 2655–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christensen LD, van Gennip M, Jakobsen TH, Alhede M, Hougen HP, et al. (2012) Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J Antimicrob Chemother 67: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 8. Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, et al. (2012) Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother 56: 2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barsi MC, Das BC, Fourrey JL, Sundaramoorthi R (1985) A concise synthesis of (±)-vincadifformine and related Aspidosperma alkaloids (±)-desethylvincadifformine, (±)-ibophyllidine, (±)-20-epi-ibophyllidine, and (±)-desethylibophyllidine. J Chem Soc Chem Commun 2 88–89. [Google Scholar]
- 10. Dupont C, Guénard D, Tchertanov L, Thoret S, Guéritte F (1999) D-ring substituted rhazinilam analogues: semisynthesis and evaluation of antitubulin activity. Bioog Med Chem 7: 2961–2970. [DOI] [PubMed] [Google Scholar]
- 11. Pandey G, Kumara CP (2011) Iminium ion Cascade reaction in the total synthesis of (+)-vincadifformine. Org Lett 13: 4672–4675. [DOI] [PubMed] [Google Scholar]
- 12. Bedu E, Benhid R, Devys M, Fourrey JL (1999) Novel 2′-deoxycytidine analogues as pH independent substitutes of protonated cytosines in triple helixformingoligonucleotides. Tetrahedron Lett 40 835–838. [Google Scholar]
- 13. Dumas C, Kan-Fan C, Royer J, Husson HP (2000) Oxidation of the CDE rings of pentacyclic quinoline compounds derived from tetrahydroalstonine. Eur J Org C 21: 3601–3606. [Google Scholar]
- 14. Boivin J, Carpentier F, Jrad R (2006) An expedient, flexible and convergent access to selectively protected 1,5-dicarbonyl compounds. Applications to the synthesis of 2,6-disubstituted pyridines and thiopyridines 10: 1664–1672. [Google Scholar]
- 15. Nwaukwa SO, Keehn PM (1982) The oxidation of alcohols and ethersusing calcium hypochlorite [Ca(OCl)2]. Tetrahedron Lett 23: 35–38. [Google Scholar]
- 16. Schwardt O, Koliwer-Brandl H, Zimmerli R, Mesch S, Rossato G, et al. (2010) Design, synthesis, biologicalevaluation, and modeling of a non-carbohydrate antagonist of the myelin-associatedglycoprotein. Bioorg Med Chem 18: 7239–7251. [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, et al. (2005) Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol 187: 1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48 (S1): 5–16. [DOI] [PubMed] [Google Scholar]
- 19. Vandeputte OM, Kiendrebeogo M, Rasamiravaka T, Stévigny C, Duez P, et al. (2011) The flavanone naringenin reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 157: 2120–2132. [DOI] [PubMed] [Google Scholar]
- 20. Kretzschmar U, Khodaverdi V, Jeoung JH, Görisch H (2008) Function and transcriptional regulation of the isocitratelyase in Pseudomonas aeruginosa . Arch Microbiol 190: 151–158. [DOI] [PubMed] [Google Scholar]
- 21. Lindsay A, Ahmer BM (2005) Effect of sdiA on biosensors of N-acylhomoserine lactones. J Bacteriol 187: 5054–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nelson BC, Putzbach K, Sharpless KE, Sander LC (2007) Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange (Citrus aurantium). J Agric Food Chem 55: 9769–7975. [DOI] [PubMed] [Google Scholar]
- 23. Shabana M, Gonaid M, Salama MM, Abdel-Sattar E (2006) Phenylalkylamine alkaloids from Stapelia hirsuta L. Nat Prod Res. 20: 710–714. [DOI] [PubMed] [Google Scholar]
- 24. Frank M, Weckman TJ, Wood T, Woods WE, Tai CL, et al. (1990) Hordenine: pharmacology, pharmacokinetics and behavioural effects in the horse. Equine Vet J 22: 437–441. [DOI] [PubMed] [Google Scholar]
- 25. Hapke HJ, Strathmann W (1995) Pharmacological effects of hordenine. Dtsch Tierarztl Wochenschr 102: 228–232. [PubMed] [Google Scholar]
- 26. Park J, Kaufmann GF, Bowen JP, Arbiser JL, Janda KD (2008) Solenopsin A, a venomalkaloid from the fireant Solenopsis invicta, inhibits quorum-sensing signaling. J Infect Dis 198: 1198–1201. [DOI] [PubMed] [Google Scholar]
- 27. Peters L, Gönig GM, Wright AD, Pukall R, Stackebrandt E, et al. (2003) Secondary metabolites of Flustrafoliacea and their influence on bacteria. Appl Environ Microbiol 69: 3469–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hosoda K, Shimomura H, Hayashi S, Yokota K, Hirai Y (2011) Steroid hormones as bactericidal agents to Helicobacter pylori . FEMS Microbiol Lett 318: 68–75. [DOI] [PubMed] [Google Scholar]
- 29. Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, et al. (2010) Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl Environ Microbiol 76: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stevens AM, Queneau Y, Soulère L, von Bodman S, Doutheau A (2011) Mechanisms and synthetic modulators of AHL-dependent gene regulation. Chem Rev 111: 4–27. [DOI] [PubMed] [Google Scholar]
- 31. Rumbaugh KP (2007) Convergence of hormones and autoinducers at the host/pathogen interface. Anal Bioanal Chem 387: 425–435. [DOI] [PubMed] [Google Scholar]
- 32. Hughes DT, Sperandio V (2008) Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soulère L, Sabbah M, Fontaine F, Queneau Y, Doutheau A (2010) LuxR-dependent quorum sensing: computer aided discovery of new inhibitors structurally unrelated to N-acylhomoserine lactones. Bioorg Med Chem Lett 20: 4355–4358. [DOI] [PubMed] [Google Scholar]
- 34. Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, et al. (2007) Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 13: 560–578. [DOI] [PubMed] [Google Scholar]
- 35. Casetta A, Audibert F, Brivet F, Boutros N, Boithias C, et al. (2003) Emergence of nosocomial Pseudomonas aeruginosa colonization/infection in pregnant women with preterm premature rupture of membranes and in their neonates. J Hosp Infect 54: 158–160. [DOI] [PubMed] [Google Scholar]
- 36. Bayó M, Berlanga M, Agut M (2002) Vaginal microbiota in healthy pregnant women and prenatal screening of group B streptococci (GBS). Int Microbiol 5: 87–90. [DOI] [PubMed] [Google Scholar]
- 37. Nisenblat V, Engel-Yeger B, Ohel G, Aronson D, Granot M (2010) The association between supra-physiological levels of estradiol and response patterns to experimental pain. Eur J Pain 14: 840–846. [DOI] [PubMed] [Google Scholar]
- 38. Rowland SS, Falkler WA Jr, Bashirelahi N (1992) Identification of an estrogen-binding protein in Pseudomonas aeruginosa . J Steroid Biochem Mol Biol 42: 721–727. [DOI] [PubMed] [Google Scholar]
- 39. Isabelle M, Villemur R, Juteau P, Lépine F (2011) Isolation of estrogen-degrading bacteria from an activated sludge bioreactor treating swine waste, including a strain that converts estrone to β-estradiol. Can J Microbiol 57: 559–568. [DOI] [PubMed] [Google Scholar]
- 40. Liang R, Liu H, Tao F, Liu Y, Ma C, et al. (2012) Genome sequence of Pseudomonas putida strain SJTE-1, a bacterium capable of degrading estrogens and persistent organic pollutants. J Bacteriol 194: 4781–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, et al. (1997) Quorum-sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143: 3703–3711. [DOI] [PubMed] [Google Scholar]
- 42. Cha C, Gao P, Chen YC, Shaw PD, Farrand SK (1998) Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol Plant Microbe Interact 11: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 43. Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, et al. (1974) Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA 71: 3672–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carlier A, Chevrot R, Dessaux Y, Faure D (2004) The assimilation of gamma-butyrolactone in Agrobacterium tumefaciens C58 interferes with the accumulation of the N-acyl-homoserine lactone signal. Mol Plant Microbe Interact 9: 951–957. [DOI] [PubMed] [Google Scholar]
- 45. Su S, Khan SR, Farrand SK (2008) Induction and loss of Ti plasmid conjugative competence in response to the acyl-homoserine lactone quorum-sensing signal. J Bacteriol 190: 4398–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phillips C, Roberts LR, Schade M, Bazin R, Bent A, et al. (2011) Design and structure of stapled peptides binding to estrogen receptors. J Am Chem Soc 133: 9696–9699. [DOI] [PubMed] [Google Scholar]
- 47. Podust LM, Yermalitskaya LV, Lepesheva GI, Podust VN, Dalmasso EA, et al. (2004) Estriol bound and ligand-free structures of sterol 14alpha-demethylase. Structure 12: 1937–1945. [DOI] [PubMed] [Google Scholar]
- 48.Thompson MA (2004) ArgusLaB 4.0.1 planetaria Software LLC Seattle WA.
- 49. Bottomley MJ, Muraglia E, Bazzo R, Carfi A (2007) Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem 282: 13592–13600. [DOI] [PubMed] [Google Scholar]
- 50. Zhang RG, Pappas KM, Brace JL, Miller PC, Oulmassov T, et al. (2002) Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417: 971–974. [DOI] [PubMed] [Google Scholar]
- 51. Ishida T, Ikeda T, Takiguchi N, Kuroda A, Ohtake H, et al. (2007) Inhibition of quorum-sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl Environ Microbiol 73: 3183–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Delden C, Pesci EC, Pearson JP, Iglewski BH (1998) Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect Immun 66: 4499–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pesci EC, Pearson JP, Seed PC, Iglewski BH (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa . J Bacteriol 179: 3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]