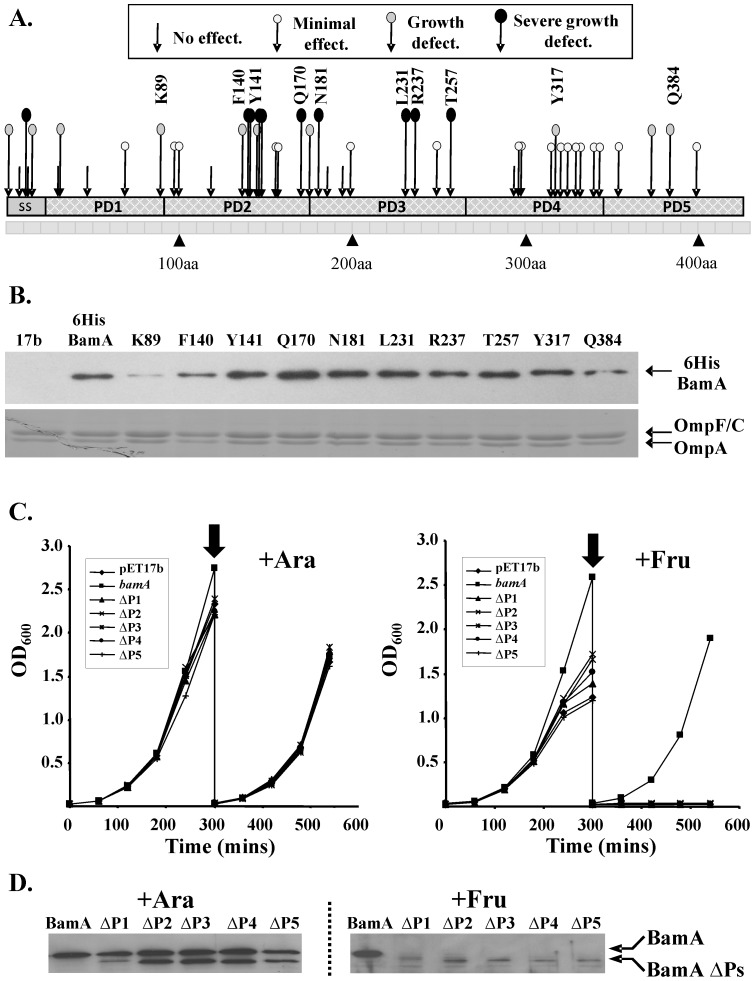
Figure 1. Mutational analysis of the BamA POTRA domains.
(A) The panel details the location of 5 amino acid insertions within the BamA POTRA domains. The BamA signal sequence (ss) and POTRA domains (PD1 to PD5) are aligned with the aa sequence number. The function of insertions was monitored by their ability to rescue BamA depletion and/or grow in the presence of vancomycin (i.e. 37.5, 75 and 150 µg ml−1). A severe growth defect (black lollipops) was defined as either an inability to rescue BamA depletion or to allow growth in the presence of vancomycin. Constructs producing a growth defect (grey lollipops) allowed growth on only 37.5 µg ml−1 vancomycin, whilst constructs which grew at 75 µg ml−1 vancomycin caused a minimal effect (white lollipops). Constructs which allowed growth at all vancomycin concentrations tested (arrows) had no effect. (B) Detection of BamA POTRA insertions. N-terminal 6His tags were introduced into the K89, F140, Y141, Q170, N181, L231, R237, T257, Y317 and Q384 insertion constructs, cloned into pET17b. Total membranes were prepared from JWD3 cells containing pET17b and the various pET17b/6hisbamA constructs, grown in the presence of arabinose. 1.6 µg of membrane protein was Western blotted with anti-6His antiserum (top) and 4 µg was analysed using SDS-PAGE and stained with Coomassie blue (bottom). (C) Deletion analysis of BamA POTRA domains. The panel shows the growth of JWD3 cells carrying pET17b, pET17b/bamA and pET17b containing bamA constructs with individual POTRA domains deleted (ΔP1 to ΔP5). Cells were grown in Lennox broth in the presence of arabinose or fructose (+Ara or +Fru). After 300 minutes cultures were sampled and subcultured into fresh medium. (D) A Western blot of normalised total cellular protein samples from JWD3 cells after 300 minutes of growth. Blots were probed with anti-BamA POTRA antiserum.