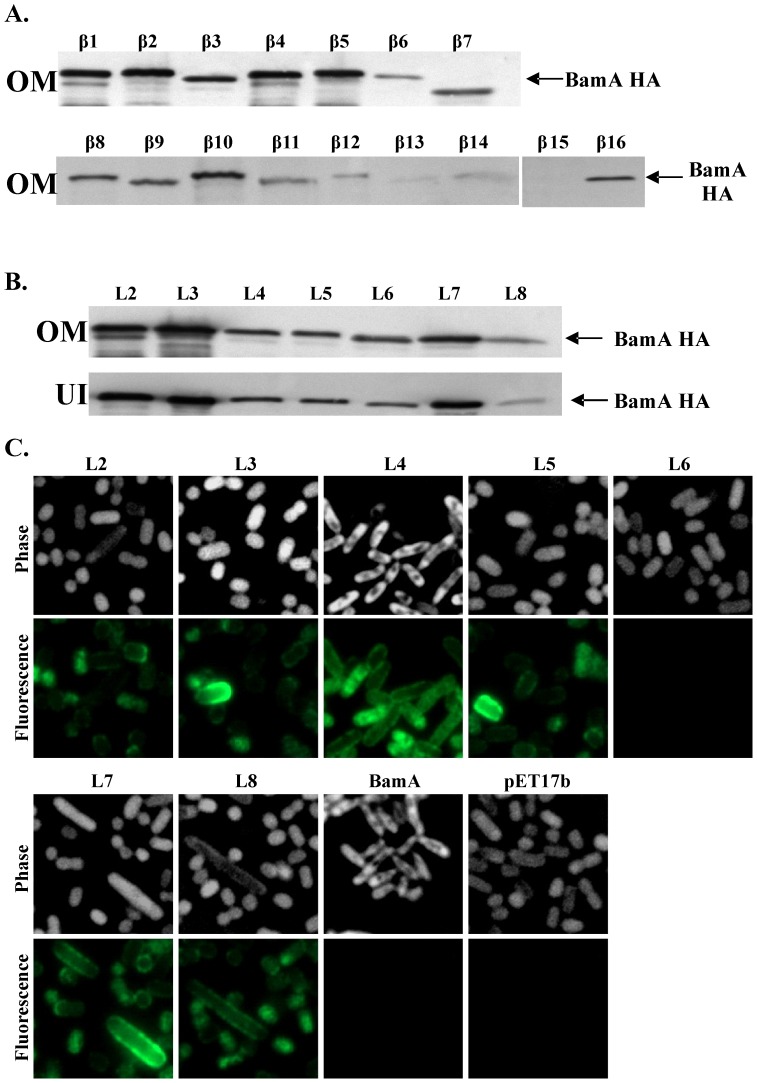
Figure 4. Analysis of HA epitopes within the BamA β-barrel.
The figure shows the detection of BamA proteins carrying HA insertions within the β-strands and external loops of the BamA β-barrel. HA epitopes were introduced into β-strands (β1 to β16) and loops (L2 to L8) (see Fig. 2) and bamA insertion constructs were cloned into pET17b. Outer membranes (OM) were prepared from JWD3 cells containing each construct and normalised protein samples were subjected to Western blotting with anti-HA antiserum. (A) shows a Western blot analysis of BamA proteins carrying HA epitopes in β-strands β1 to β16 and (B), in loops L2 to L8. Panel (B) also shows the urea insoluble fraction (UI) obtained after outer membrane preparations were washed with urea. All BamA proteins carrying HA epitopes in their loop domains were localised within the urea insoluble fraction, indicating that they are correctly folded within the membrane. (C) Immunofluorescence analysis of BamA constructs carrying HA epitopes within external loops. JWD3 cells, containing pET17b, pET17b/bamA or pET17b carrying HA insertions in L2 to L8, were fixed, probed with anti-HA and Alexa Fluor® 488 antibody and visualized using phase contrast (shown inverted) and fluorescence microscopy.