Abstract
Psychopathy is a disorder characterized by reduced empathy, shallow affect and behaviors that cause victims distress, like threats, bullying and violence. Neuroimaging research in both institutionalized and community samples implicates amygdala dysfunction in the etiology of psychopathic traits. Reduced amygdala responsiveness may disrupt processing of fear-relevant stimuli like fearful facial expressions. The present study links amygdala dysfunction in response to fear-relevant stimuli to the willingness of individuals with psychopathic traits to cause fear in other people. Thirty-three healthy adult participants varying in psychopathic traits underwent whole-brain fMRI scanning while they viewed statements that selectively evoke anger, disgust, fear, happiness or sadness. During scanning, participants judged whether it is morally acceptable to make each statement to another person. Psychopathy was associated with reduced activity in right amygdala during judgments of fear-evoking statements and with more lenient moral judgments about causing fear. No group differences in amygdala function or moral judgments emerged for other emotion categories. Psychopathy was also associated with increased activity in middle frontal gyrus (BA 10) during the task. These results implicate amygdala dysfunction in impaired judgments about causing distress in psychopathy and suggest that atypical amygdala responses to fear in psychopathy extend across multiple classes of stimuli.
Keywords: psychopathy, amygdala, moral judgment, fear, empathy, antisocial
Psychopathy is a disorder characterized by antisocial behavior, reduced empathy and remorse, narcissism and impulsivity (Hare, 1991). Elevated psychopathic traits have been linked to atypical neural functioning in brain structures that include the amygdala, orbitofrontal cortex and striatum (Birbaumer et al., 2005; Rilling et al., 2007; Dolan and Fullam, 2009; Jones, et al., 2009; Harenski, et al., 2010; Finger et al., 2011; Marsh et al., 2011a). These disruptions are thought to underlie emotion processing deficits in psychopathy, particularly disruptions in recognizing and responding to fear-relevant stimuli such as fearful facial expressions (Marsh and Blair, 2008). A lingering central question is how these neural and cognitive disruptions increase psychopathic individuals’ risk for engaging in antisocial behaviors such as threats, bullying and instrumental violence (Skeem et al., 2011). The present study aimed to address this question using a novel task in which healthy adult participants varying in psychopathic traits reasoned about the moral acceptability of causing people to experience fear, as well as other emotions like anger and disgust (Marsh and Cardinale, 2012).
Psychopathy has long been known to impair recognition of and responses to fear-related stimuli. Fear-processing deficits in psychopathy include reduced electrodermal responses, potentiated startle, Pavlovian conditioning and passive avoidance to impending threats like shock (Blair, 2005), as well as impaired recognition of fear expressed by the face (Marsh and Blair, 2008; Sylvers, et al., 2011), voice (Blair et al., 2002, 2005) and body (Munoz, 2009). Psychopathy is also linked to reduced subjective experience of fear, which has been described anecdotally (Hare, 1993) as well as empirically (Aniskiewicz, 1979; Lykken, 1995; Birbaumer et al., 2005; Marsh et al., 2011b). Therefore, although many descriptions of psychopathy emphasize general poverty of affect, psychopathy appears to disrupt fear more than other emotions among both institutionalized and community samples.
Amygdala dysfunction has been identified as a likely source of deficient processing of fear-relevant stimuli in psychopathy (Kiehl et al., 2001; Blair, 2007; Shirtcliff et al., 2009; Yang et al., 2009). The amygdala is essential for evaluating the emotional significance of stimuli, particularly fear-relevant stimuli (LeDoux, 2003; Whalen, 2007). Two meta-analyses of human neuroimaging data have shown that the amygdala responds preferentially to fear stimuli, including fearful facial expressions, relative to other categories of emotional stimuli (Phan et al., 2002; Murphy et al., 2003). Supporting the idea that the amygdala is critical for fear responding, amygdala lesions result in impaired fear conditioning, potentiated startle and recognition of fearful facial expressions (Adolphs et al., 1994; LaBar et al., 1995; Angrilli et al., 1996). Because psychopathy impairs performance in these same tasks, it is hypothesized that impaired fear responding in psychopathy results from amygdala dysfunction. Consistent with this, psychopathy is associated with reduced amygdala activity during exposure to fear-relevant stimuli like fearful facial expressions but not, for example, angry or neutral expressions (Marsh et al., 2008; Dolan and Fullam, 2009; Jones et al., 2009).
Is this ‘fear blindness’ relevant to the fact that individuals with psychopathic traits engage in behaviors that cause others fear, including threats, bullying and violence (Blair et al., 2006)? Research conducted by Marsh and Cardinale (2012) recently linked these features of psychopathy in a community sample. Paralleling prior findings that psychopathy impairs the ability to identify which emotional expressions convey fear, psychopathy was found to impair the ability to identify which of a series of emotionally evocative statements would cause fear in a listener. Moreover, psychopathy resulted in more lenient moral judgments about the acceptability of causing someone fear. And impaired recognition of the fear-evoking statements predicted leniency of judgments. These deficiencies were specific to fear; judgments of other types of transgressions were largely unrelated to psychopathy.
These data suggest that fear-processing deficits in psychopathy extend to judgments about causing others fear. The present study assesses the role of the amygdala in this feature of psychopathy. We conducted whole-brain fMRI scanning in healthy adults who varied in psychopathic traits as they judged the moral acceptability of emotionally evocative statements. We first wished to determine whether high psychopathy scorers would show atypical amygdala activity only when evaluating the acceptability of causing fear. We predicted that low psychopathy scorers would show increased amygdala activation when judging fear-evoking statements and that high psychopathy scorers would not show this increase. Behaviorally, we predicted that reduced amygdala activation in psychopathy would be accompanied by more lenient moral judgments about causing fear. In keeping with prior findings, we also predicted that psychopathy would be associated with increased activity in dorsolateral prefrontal cortex across multiple categories of moral judgments (Rilling et al., 2007; Glenn et al., 2009a).
METHODS
Participants
Thirty-three adult volunteers (20 females, 13 males, M age = 22.0 years, s.d. = 1.8 years, range = 18–25 years) were recruited from the Georgetown University community and underwent fMRI scanning. High psychopathy scorers were oversampled using recruitment advertisements developed for use in psychopathy research (Widom, 1977). All participants were right-handed and free of psychotropic medications. Participants were screened for current diagnosis of and treatment for mental illness and no participant reported either current diagnosis or treatment. The mean IQ of the sample measured using the K-BIT (Kaufman, 1990) was 113.2 (s.d. = 15.6, range = 80–143). The study was approved by the Institutional Review Board at Georgetown University, and all participants provided informed written consent in accordance with the Declaration of Helsinki.
Measurement of psychopathy and aggression
Psychopathy was measured using the Psychopathic Personality Inventory-Revised (PPI-R; Lilienfeld and Widows, 2005). The PPI-R is a 154-item self-report measure designed to measure psychopathic traits in a dimensional manner, consistent with indications that psychopathy can be more accurately assessed dimensionally than taxonomically (Skeem et al., 2011). Items were developed in accordance with Cleckley’s original characterization of psychopathy (Cleckley, 1988), and each item consists of a statement to which participants indicate how accurately it applies to them using a 4-point scale with response options ranging from 1 ‘false’ to 4 ‘true’. The PPI-R shows relations with psychopathy-relevant criterion measures that parallel those for other measures of psychopathy, notably the PCL-R, which is predominantly used to assess psychopathy in institutionalized samples (Poythress et al., 2010). The revised PPI and its predecessor have been successfully used in many previous neuroimaging explorations of cognitive and moral dysfunction in psychopathy (Gordon et al., 2004; Nunez et al., 2005; Fullam et al., 2009). Among the PPI-R subscales are Fearless Dominance, which indexes primarily affect and empathy deficits relevant to psychopathy (akin to Factor 1 of the PCL variants) and Self-Centered Impulsivity, which indexes impulsive antisociality (Factor 2). Participants were also administered the adult form of the Reactive-Proactive Aggression Questionnaire (RPQ; Raine et al., 2006) a self-report measure that assesses aggressive behavior, including both reactive and proactive aggression.
Emotionally evocative statements task
The Emotionally Evocative Statements Task (EEST) was adapted from the task previously described by Marsh and Cardinale (2012). The task incorporates 100 emotionally evocative statements (see Table 1 for sample statements), which are an ecologically valid and sensitive means of eliciting specific emotional responses during neuroimaging (Blair et al., 2008). The statements were generated by young adult university students (comparable to the population tested in this study) in response to a request for statements a person could make to cause another to feel anger, disgust, fear, happiness or sadness. Statements were edited to clarify meaning and to equalize word count among the five emotion categories (Ps > 0.10). A separate sample of participants rated the emotional evocativeness of each statement, and the final statements selected as task stimuli were those that elicited maximal emotion category agreement across participants. Specifically, we calculated four t-values that compared the magnitude of each statement’s mean rating for the intended emotion compared to the other four emotions and selected the 20 statements from each category with the highest average t-values (mean t-values: anger = 6.78, disgust, = 10.96, fear = 7.45, happiness = 52.11, sadness = 9.79). Overall agreement as to the emotions generated by the statements is high on average (M all emotions = 81.5%; anger = 56%; disgust = 93%; fear = 75%; happiness = 99%; sadness = 84%) and comparable to picture-based emotion recognition tasks (Britton et al., 2006). Twenty affectively neutral statements were also included as a baseline condition for neuroimaging.
Table 1.
Sample stimuli
| Anger | Sadness | Fear | Happiness | Neutral | Disgust |
|---|---|---|---|---|---|
| ‘I told you to shut up.’ | ‘I’m not attracted to you.’ | ‘I could easily hurt you.’ | ‘You always make me smile.’ | ‘I’m going to take a nap.’ | ‘I never wash my hands.’ |
| ‘You are a disgrace.’ | ‘I have no respect for you.’ | ‘You can’t protect yourself from me.’ | ‘I love you.’ | ‘I saw a movie last night.’ | ‘I haven’t showered in days.’ |
fMRI scanning task
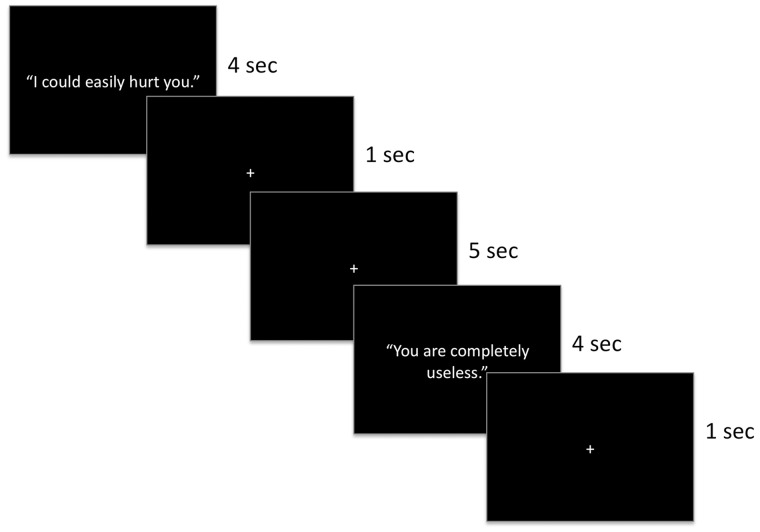
During the scan session, four event-related fMRI runs were acquired from each participant, during which participants viewed emotionally evocative statements from the EEST that were projected onto a screen they viewed in a mirror in the MRI scanner. Participants rated the moral acceptability of making each statement to another person. Prior to the commencement of scanning, participants were placed in a light head restraint within the scanner to reduce movement and were instructed to read each statement that appeared on the screen and decide whether it would ever be morally acceptable to make that statement to another person. Responses were collected using response buttons held in the left and right hand, which participants pressed with their thumbs and corresponded to answers of ‘yes’ and ‘no’, respectively. Responses were therefore binary rather than ordinal points on a scale. The order in which the four runs of the task were completed was counterbalanced across participants.
During each of the runs, five statements from each of the six emotion categories were presented for 4000 ms each, during which time participants’ responses were collected. Each run used a separate stimulus set and the order in which the statements in each run were presented was randomized. Each statement slide was followed by a 1000-ms fixation cross. In addition, 10 fixation trials (jitters) appeared for 5000 ms at random intervals during each run of the task. Each run began and concluded with three 5000-ms baseline fixation trials. The task was programmed in E-Studio.
T2* weighted images were collected during fMRI scanning using a 3.0 Tesla Siemens (Erlangen, Germany) TIM Trio whole-body MRI system with echo planar imaging capability (matrix 64 × 64; repetition time, 2500 ms; echo time, 30 ms; field of view, 192 mm; voxels, 3.0 × 3.0 × 3.0 mm). Functional images were acquired with a gradient echo-planar imaging (EPI) sequence (axial plane, 46 interleaved slices). High-resolution T1-weighted anatomical images were also acquired (3D Magnetization Prepared Rapid Acquisition Gradient Echo; 176 1.0-mm axial slices; 250 mm field of view; 1900 ms repetition time, 2.52 ms echo; 246 × 256 matrix).
Preprocessing of fMRI data
Data were analyzed within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI; Cox, 1996). Both individual and group-level analyses were conducted. The first four volumes in each of the four scan series, collected before magnetization equilibrium was reached, were discarded, leaving 88 TRs per run and 352 TRs total per participant. Data were then concatenated, despiked, motion corrected, spatially smoothed using a 6.0-mm full-width half-maximum Gaussian filter and activation outside the brain was masked. The time series was then normalized such that signal amplitude and regression coefficients represent a percent signal change from the mean. Regressors were created for each emotion category (anger, disgust, fear, happiness, neutral and sadness), and a regressor of no interest was created for trials in which participants did not provide a response. Fixation trials were modeled implicitly. The train of stimulus events was then convolved with a γ-variate hemodynamic response function to account for the slow hemodynamic response (Cohen, 1997). The baseline was modeled by a first-order function and motion artefacts were modeled using the six estimated rigid-body motion parameters. This produced a β-coefficient and associated t-statistic for each voxel and regressor. Participants’ anatomical scans were individually registered to the Talaraich and Tournoux Atlas (Talairach and Tournoux, 1988). Following normalization, voxels measured 3 mm3.
Statistical analysis
Participants were divided into high and low psychopathy scorers on the basis of a median split. For the analysis of behavioral data, participants’ judgments (which were dichotomous) were converted to ratios of unacceptable to acceptable ratings such that higher scores reflect less acceptance. Judgments were analyzed using a 2 (group: low psychopathy and high psychopathy) × 6 (emotion: anger, disgust, fear, happiness, neutral and sadness) repeated-measures ANOVA. Planned t-tests compared responses to each emotion category across high and low psychopathy scorers. Main effects and interactions are reported at P < 0.05, two tailed.
To identify whether activation in the hypothesized brain regions is differentially associated with judgments of the emotion categories as a function of psychopathy, we used two analytic strategies for fMRI data. Hypothesized areas of differential activation that survive both analytic strategies are reported. First, to identify whether a true group × emotion interaction existed in the hypothesized regions we conducted a whole-brain 2 (group: low psychopathy and high psychopathy) × 6 (emotion: anger, disgust, fear, happiness, neutral and sadness) random-effects ANOVA that paralleled our behavioral analysis. ANOVA results were reported that exceeded P < 0.005, uncorrected, for magnitude, with an extent threshold of 10 contiguous voxels, a joint thresholding procedure that balances the risk of type I and type II errors, (Lieberman and Cunningham, 2009), a critical consideration for omnibus interactions (Wahlsten, 1990). Small volume correction (SVC) was applied to clusters located in the amygdala. Mean parameter estimates were extracted from functionally defined regions of interest (ROIs) resulting from the ANOVA to determine the nature of the interaction and to assess the relationship between patterns of neural activation and patterns of behavior (aggression scores). Second, we performed focused tests of our hypothesis, calculated independently from the ANOVA, by conducting five whole-brain double-subtraction contrasts within AFNI (emotion − neutral, low psychopathy − high psychopathy). For these contrast tests, we applied a threshold that yielded a whole-brain false discovery rate (FDR) < 0.05 across all five contrasts (P < 0.0001, uncorrected, with an extent threshold of 10 voxels).
RESULTS
Psychopathy and aggression scores
Consistent with our goal to oversample psychopathic traits, the mean PPI-R score in our sample was 305.4 (s.d. = 41.21, range = 232–412), higher than would be anticipated in a typical community sample of 18- to 25-year-old females and males and higher than that reported in a male offender sample tested during development of the PPI-R (Lilienfeld and Widows, 2005). Following the median split, the mean score for high psychopathy scorers was 337.29 (s.d. = 33.39) and for low psychopathy scorers it was 275.45 (s.d. = 19.52). High psychopathy scorers reported engaging in more aggressive behavior (M = 10.27, s.d. = 6.03) than low scorers (M = 5.94, s.d. = 3.34), t(31) = 2.57, P < 0.05. No group differences emerged for distribution of sex, IQ or age across psychopathy groups (all Ps > 0.20).
Behavioral data
Participants’ moral acceptability judgments during the EEST varied across emotion categories, F(5, 135) = 16.598, P < 0.001. Participants judged causing others to feel fear (M = 4.105, s.d. = 4.31) and anger (M = 4.732, s.d. = 6.11) as least acceptable; ratings of these emotions were not significantly different, t(32) = 1.22, P = 0.233. These judgments were followed by judgments of statements that evoke disgust (M = 0.95, s.d. = 1.85), sadness (M = 0.77, s.d. = 0.91) and happiness (M = 0.02, s.d. = 0.05) and neutral statements (M = 0.02, s.d. = 0.07). There was no main effect of psychopathy group across emotion categories, F(1, 27) = 1.828, P = 0.187. The interaction between emotion and psychopathy group was marginally significant, F(5, 140) = 2.076, P = 0.072. To specifically test our behavioral hypothesis that only judgments of fear-evoking statements would differ across groups, we then collapsed participants’ responses to all non-fear statements and calculated a 2 (group) × 2 (fear statements, average of all other statements) ANOVA, the results of which yielded a similar interaction effect, F(1, 31) = 3.722, P = 0.063. The nature of the interaction was such that high psychopathy scorers judged causing others fear to be more acceptable (M = 2.51, s.d. = 1.67) than low psychopathy scorers did (M = 5.61, s.d. = 2.58), t(30) = 2.190, P < 0.05, whereas no group differences emerged for non-fear statements, t(30) = 1.333, P = 0.192, consistent with previous findings (Marsh and Cardinale, 2012) (Table 2).
Table 2.
Average acceptability judgments for high and low psychopathy scorers
| Emotion | Low psychopathy | High psychopathy | t value |
|---|---|---|---|
| Fear | 5.61 | 2.51 | 2.19* |
| All non-fear | 1.59 | 0.94 | 1.33 |
| Anger | 6.47 | 3.09 | 1.59 |
| Disgust | 0.91 | 0.99 | 0.14 |
| Happiness | 0.01 | 0.02 | 0.40 |
| Neutral | 0.00 | 0.03 | 1.45 |
| Sadness | 1.00 | 0.54 | 1.46 |
*P < 0.05 (two tailed).
fMRI data
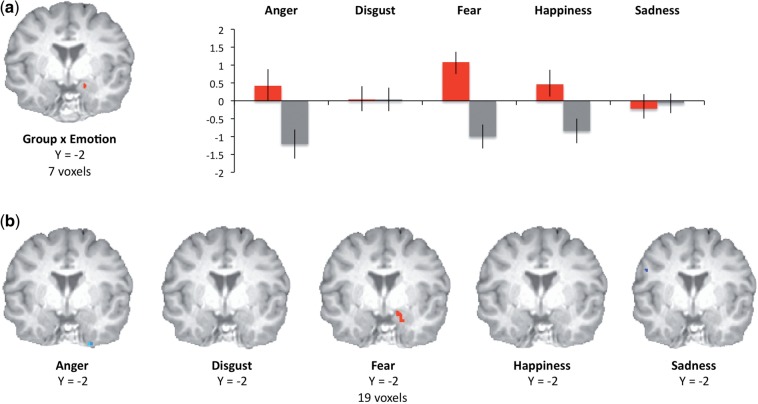
The results of the group × emotion interaction identified a cluster of differential activation in right amygdala/parahippocampal gyrus, F(5, 135) = 3.498, P < 0.005, SVC (xyz = 14 8 −19). Analyses of mean parameter estimates within this functionally defined ROI confirmed that fear was also the only emotion for which low psychopathy scorers showed significantly greater activation than high scorers, t(31) = 3.738, P < 0.001 and that, for low psychopathy scorers, fear was the only emotion for which amygdala activity significantly exceeded baseline (neutral condition), t(16) = 3.732, P < 0.005 (all other Ps > 0.20). For high psychopathy scorers, in no condition did activation in this ROI exceed neutral condition activation; activation was significantly reduced relative to neutral in high psychopathy scorers for anger, fear and happiness (Ps < 0.05) (Figure 1a).
Fig. 1.
Neuroimaging task design and stimulus presentation.
The results of five independent double-subtraction analyses calculated in AFNI (such that differences in activation from the neutral condition for each type of emotion were compared across high and low psychopathy scorers) supported the specificity of this effect: fear was the only emotion category for which group differences emerged in the amygdala during the task (xyz = 14 − 4 − 19) (Figure 1b).
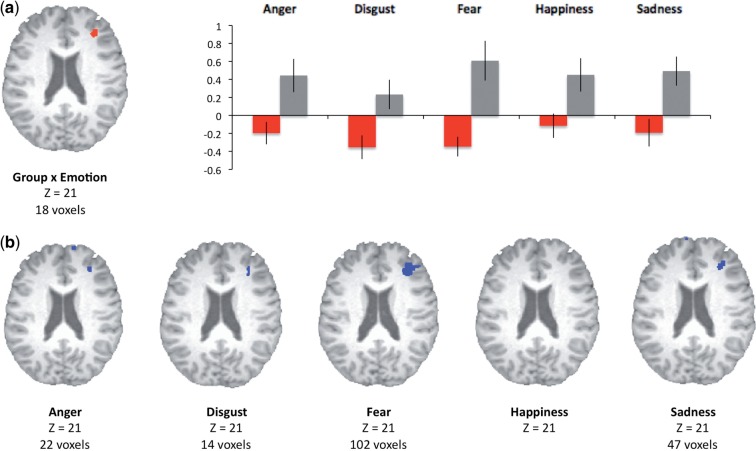
Our ANOVA also identified a group × emotion interaction effect in right middle frontal gyrus (BA 10, xyz = 35, 38, 20). Analyses of mean parameter estimates confirmed that, for high psychopathy scorers, activation in this region exceeded baseline for multiple emotion categories (Ps < 0.05 for all emotions except disgust, P = 0.18). By contrast, activation in this region did not significantly exceed baseline for any emotion in low psychopathy scorers (Figure 2a). The results of double-subtraction analyses computed in AFNI confirmed increased activation in this region in high psychopathy scorers across multiple emotions (Figure 2b).
Fig. 2.
Clusters in which differential activation was observed in high psychopathy scorers (gray bars) and low psychopathy scorers (red bars) following a whole-brain 2 (group) × 6 (emotion category) ANOVA. Mean (SEM) percent signal changes extracted from functionally identified ROIs in the amygdala are presented at right (a). Clusters in which differential activation was observed in high psychopathy scorers and low psychopathy scorers following double-subtraction analyses (fear − neutral, low psychopathy − high psychopathy) across emotion categories in right amygdala (b).
The results of our omnibus ANOVA identified group × emotion interaction effects in only two regions in addition to right amygdala and right middle frontal gyrus: left and right anterior precuneus (BA 7, xyz = −7 −61 47 and 2 −55 44, Table 2). No other interaction effects or main effects of group were identified (main effects of emotion can be seen in Table 3). Post hoc analyses of mean parameter estimates indicated that, bilaterally, low psychopathy scorers showed the greatest activation in this region (relative to neutral) when judging fear-based transgressions, whereas high psychopathy scorers showed the least activation in this region when judging fear-based transgressions (Figure 3). When raw means for each emotion category were compared across groups, activation for low psychopathy scorers exceeded that for high psychopathy scorers only during the fear (right P < 0.005, left P < 0.005) and neutral condition (left P < 0.01, right P < 0.05).
Table 3.
Functionally defined ROIs identified by a group [high psychopathy, low psychopathy × emotion (anger, disgust, fear, happiness and neutral sadness)] ANOVA in AFNI
| Cluster | BA | X | Y | Z | Voxels |
|---|---|---|---|---|---|
| Group × emotion | |||||
| R middle frontal gyrus | 10 | +35 | +38 | +21 | 18 |
| R amygdala/parahippocampal gyrus | +14 | +8 | −19 | 7 | |
| R precuneus | 7 | +2 | −56 | +45 | 13 |
| L precuneus | 7 | −8 | −62 | +48 | 13 |
| Emotion | |||||
| R middle frontal gyrus | 11 | +17 | +38 | −13 | 13 |
| R cingulate gyrus | 32 | +2 | +29 | +27 | 20 |
| L inferior frontal gyrus | 45 | −47 | +20 | +3 | 16 |
| R precentral gyrus | 6 | +41 | −17 | +60 | 21 |
| R precentral gyrus | 4 | +35 | −29 | +63 | 39 |
| R postcentral gyrus | 3 | +56 | −23 | +42 | 41 |
| L caudate (tail) | −35 | −26 | −4 | 17 | |
| R precuneus, | 7 | 14 | −53 | +54 | 19 |
| L precuneus | 7 | −8 | −53 | +54 | 10 |
| R cuneus | 18 | +5 | −71 | +18 | 11 |
| R cuneus | 19 | +5 | −92 | +33 | 10 |
| Group | |||||
| – |
Fig. 3.
Clusters in which differential activation was observed in high psychopathy scorers (gray bars) and low psychopathy scorers (red bars) following a whole-brain 2 (group) × 6 (emotion category) ANOVA. Mean (SEM) percent signal changes extracted from functionally identified ROIs in dorsolateral prefrontal cortex is presented at right (b). Clusters in which differential activation was observed in high psychopathy scorers and low psychopathy scorers following double-subtraction analyses (fear − neutral, low psychopathy − high psychopathy) across emotion categories in right middle frontal gyrus (a).
To estimate the relationship between activation patterns during task performance and self-reported aggressive behavior, we calculated the relationship between activation in the functional ROIs defined by our group × emotion interaction and aggression, as defined by RPQ scores. Results indicated that aggression was most consistently associated with neural responding during the fear condition: more aggressive participants exhibited more activation in middle frontal gyrus and less activation in left precuneus and (at the trend level) amygdala during judgments of fear-evoking statements (Table 4).
Table 4.
Correspondence between aggression (RPQ score) and neural activity in functionally defined ROIs
| Region of interest | Anger | Disgust | Fear | Happiness | Neutral | Sadness |
|---|---|---|---|---|---|---|
| R Middle frontal gyrus | 0.11 | 0.03 | 0.34* | 0.07 | 0.20 | 0.01 |
| R amygdala/parahipp. | −0.27 | 0.18 | −0.28† | 0.18 | 0.22 | 0.18 |
| R precuneus | 0.15 | 0.03 | −0.27 | 0.34* | 0.06 | 0.22 |
| L precuneus | 0.30† | 0.25 | −0.37* | 0.11 | −0.04 | 0.24 |
*P < 0.05 (two tailed).
†P ≤ 0.10 (two tailed).
Finally, we conducted post hoc analyses assessing the extent to which PPI-R factor scores related primarily to affect and empathy (Factor 1) or to impulsive antisociality (Factor 2) better predicted observed patterns of activation in the clusters generated by our group × emotion interaction. These calculations were not intended to assess absolute correlation values, but rather to assess the relative contributions of Factor 1 and Factor 2 scores to the parameter estimates. For each cluster, we calculated linear regressions in which mean parameter estimates derived in AFNI were our outcome variables and factor scores were our predictor variables. Results for the amygdala, F(2,32) = 6.539, P < 0.005, indicated that Factor 1 scores (P < 0.05) better predicted amygdala activation in this cluster than did Factor 2 scores (P = 0.298). For middle frontal gyrus, the model was not significant and neither factor significantly predicted activation. For left precuneus, F(2,32) = 8.039, P < 0.001, Factor 1 scores (P < 0.05) again predicted activation patterns better than Factor 2 scores (P = 0.491). For right precuneus, F(2,32) = 12.061, P < 0.001, both Factor 1 and Factor 2 scores predicted activation (Ps < 0.05).
DISCUSSION
Psychopathy has long been associated with an increased willingness to engage in behaviors that cause fear in victims, like threats, bullying and violence (Blair, 2005). The present study demonstrated that psychopathy is associated with aberrant amygdala responding during judgments about causing others fear. Participants in this study judged the moral acceptability of making fear-evoking statements, including threats such as ‘I could easily hurt you’ and ‘You can’t protect yourself from me’. Replicating previous results, participants with higher psychopathy scores judged making statements like these to be more acceptable (Marsh and Cardinale, 2012). This study extends these findings by demonstrating that, during their judgments, participants with high psychopathy scores showed reduced amygdala activity relative to participants with lower psychopathy scores.
These results are consistent with the modern construct of psychopathy, initially developed by Cleckley, who, in describing the prototypical psychopath, stated, ‘In the disaster he brings about he cannot estimate the affective reactions of others which are the substance of the disaster… the real psychopath seems to lack understanding of the nature and quality of the hurt and sorrow he brings to others’ (Cleckley, 1988, p. 322). Neurocognitive models of psychopathy developed more recently have built upon this idea. For example, Blair and colleagues have developed a model of violence inhibition in which impaired functioning in amygdala and associated structures (e.g. orbitofrontal cortex) disrupts appropriate emotional responding to victims’ distress, which increases the likelihood of causing ‘hurt and sorrow’ in future victims (Blair, 2005, 1997). This theory is consistent with the present findings, in which we observed reduced amygdala activation in high-psychopathy participants as they considered the acceptability of causing victims distress. In addition, high-psychopathy participants judged causing victims distress to be more acceptable and reported higher levels of actual aggressive and violent behavior.
The present results also extend previous findings on the role of the amygdala in processing fear-relevant stimuli. The results of two meta-analyses have shown that the amygdala is generally more active during the presentation of fear-relevant stimuli than other categories of emotional stimuli (Phan et al., 2002; Murphy et al., 2003) and that individuals with amygdala lesions show impairments in processing a variety of fear-relevant stimuli, including fearful facial expressions (Adolphs et al., 1994) and vocal expressions (Scott et al., 1997). But to our knowledge this is the first study to find that the amygdala is more sensitive to written statements that evoke fear relative to other emotions. In addition, it confirms that amygdala activation in response to fear-relevant stimuli increases only for individuals on the lower end of the psychopathy continuum.
Because it incorporates written stimuli linked to multiple specific emotions, the present paradigm may provide data relevant to ongoing debates about the role of the amygdala in processing fear-relevant stimuli and how amygdala dysfunction in psychopathy impairs fear processing. One prominent theory is that amygdala dysfunction impairs judgments about fearful facial expressions in psychopathy by reducing respondents’ attention to the eyes of fearful faces (Dadds et al., 2006; Han et al., 2012). This theory is supported by findings that, in both patients with amygdala lesions and in children with psychopathic traits, instructing participants to attend to the eyes of faces reduces fear recognition deficits (Adolphs et al., 2005; Dadds et al., 2008). It is argued that the eyes of fearful faces are most diagnostic features of the expression, and the amygdala helps direct attention to the most salient features of the face.
It is less clear how this theory can be extended to explain impairments in processing of other forms of fear-relevant stimuli. Amygdala lesions and psychopathy both also impair recognition of vocalized fear (Scott et al., 1997; Blair et al., 2002), auditory stimuli for which the relevance of attention directed to salient features is unclear. And a recent study shows that psychopathy also impairs pre-attentive recognition of fearful faces (Sylvers, et al., 2011). Along the same lines, the present task stimuli consist of written statements for which visual attention is not obviously relevant to recognizing the emotional consequences of the statements or making moral judgments about them. No low-level stimulus features of fear-evoking statements distinguish them from other categories of statements. Yet the influence of psychopathy on responses to fear-evoking statements in the present task precisely mirrors its influence on responses to fearful facial expressions in community and institutionalized sample—in both cases, specific deficits in identifying fear-relevant stimuli are observed (Marsh and Blair, 2008). And the reduced amygdala response to fear-evoking stimuli in high-psychopathy participants mirrors previous findings of reduced amygdala responses to fearful faces in participants with psychopathic traits (Marsh et al., 2008; Dolan and Fullam, 2009; Jones et al., 2009).
This suggests the possibility of deeper emotional processing impairments that affect how fearful stimuli as a class are processed. One possibility is that correctly identifying fearful expressions requires the generation of an internal representation of fear, which relies on intact amygdala function (Goldman and Sripada, 2005). Amygdala dysfunction in psychopathy may impede the ability to generate an internal representation of fear, thereby impairing identification of others’ fear across contexts. So, for example, correctly identifying a statement like, ‘I could easily hurt you’, as one that would evoke fear may also require the ability to create an internal representation of the target’s likely emotional response. Given previous evidence that psychopathy impairs the ability to identify fear-evoking statements (Marsh and Cardinale, 2012), and current evidence for amygdala dysfunction when reading these statements in high psychopathy scorers, our present findings are consistent with the possibility that representation of the emotional outcome of fear-evoking statements is supported by activity in the amygdala. Our previous findings underscore the previously hypothesized link between awareness that a statement causes fear and judging it to be unacceptable, supporting the possibility that both capacities may rely on the internal representation of the statement’s emotional outcome (Nichols, 2001; Pfaff et al., 2008).
The pattern of activity we observed in bilateral anterior precuneus during the task also supports the possibility that identifying and judging fear-evoking statements requires the generation of an internal representation of fear. Because this effect was not hypothesized, these results are interpreted with some caution. The anterior precuneus, termed ‘the mind’s eye’ in early imaging studies (Fletcher et al., 1995), is thought to play a central role in a variety of tasks associated with the episodic retrieval of polymodal imagery and first-person perspective taking (Cavanna and Trimble, 2006). Activation in this region increases during tasks that require third-person perspective simulation or empathizing and may reflect the generation of vivid representations of the self to imagine the internal state of another person (Ochsner et al., 2004; Ruby and Decety, 2004; Farrow et al., 2005). The results of our analyses indicated that bilateral activation in this region was greatest in participants with lower psychopathy scores evaluating fear-evoking statements, whereas the opposite was true for high-psychopathy scorers. This is consistent with the possibility that the task requires participants to draw on stored representations involving episodic emotional memories. In typical individuals frightening memories are vivid and easily retrieved (McGaugh et al., 1996), whereas this may be less evident among individuals with psychopathic traits.
Participants with high psychopathy scores showed increased activation in middle frontal gyrus (dorsolateral prefrontal cortex) when judging multiple varieties of emotional statements. This is consistent with previous psychopathy studies in which participants have made morally relevant decisions in tasks featuring trolley-car dilemmas (Glenn et al., 2009b) or prisoner’s dilemmas (Rilling et al., 2007). In both studies, increased dorsolateral prefrontal activation was observed when participants with higher psychopathy scores reasoned about the morally appropriate decision. Similar patterns of increased dorsolateral activity have also been observed during simpler emotion judgments in both high-psychopathy community samples (Gordon et al., 2004) and criminal samples (Kiehl et al., 2001). This suggests that psychopathic traits may leave individuals to rely on abstract reasoning processes when making some moral judgments because they lack the appropriate emotional input (Glenn et al., 2009b).
Fig. 4.
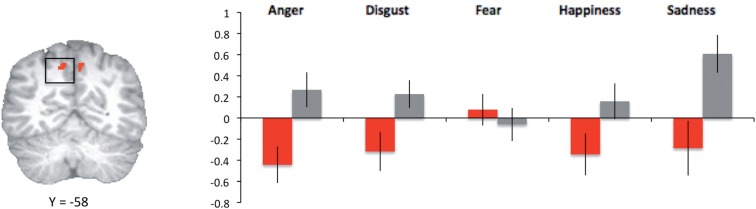
Clusters in which differential activation was observed in high psychopathy scorers (gray bars) and low psychopathy scorers (red bars) following a whole-brain 2 (group) × 6 (emotion category) ANOVA. Mean (SEM) percent signal changes extracted from functionally identified ROIs in left precuneus is presented at right.
The post hoc analyses we conducted to assess the correspondence between psychopathy factor scores and activation in our clusters of interest generally support the conclusion that the patterns we observed are related to core features of psychopathy such as shallow affect and reduced empathy. Factor 1 scores, which reflect emotional responsivity, were more closely related than Factor 2 scores to activation in amygdala and left precuneus when participants considered the moral acceptability of frightening others. Both Factor 1 and 2 scores independently predicted activation in right precuneus. Middle frontal gyrus was the only region in which activation was not significantly associated with Factor 1 scores (and were also not significantly associated with Factor 2 scores). This is interesting in light of mixed prior evidence regarding activation in middle frontal gyrus during socioemotional tasks, with some finding stronger associations with Factor 2 scores (Rilling et al., 2007; Glenn et al., 2009b) and others with Factor 1 scores (Gordon et al., 2004). One possibility is that involvement of this region among high psychopathy scorers is strongly task specific.
It should be noted that it is unknown whether the findings from our high psychopathy scorers from a community sample would extend to criminal psychopaths. Accumulating evidence supports the quantitatively continuous nature of psychopathy in the population, rather than ‘psychopaths’ being a taxon who differ qualitatively from individuals with lower scores (Skeem et al., 2011). In this sense, psychopathy may be much like other forms of psychopathology, which a recent meta-analysis confirms can be assessed more accurately and reliably when they are treated as continuous rather than discrete measures (Markon et al., 2011). And high-psychopathy participants in our sample obtained objectively high scores on the PPI-R, comparable to scores previously obtained in a criminal sample (Lilienfeld and Widows, 2005). That said, the present results should be replicated with samples that are assessed using other measures of psychopathy, such as one of the Psychopathy Checklist (PCL) variants, which are the dominant means of assessing psychopathy in institutionalized research samples (Skeem and Cooke, 2010), or the Levenson Self-Report Psychopathy measure, which in at least one prior study was found to be a better predictor than the PPI-R of neural activation patterns (Rilling et al., 2007).
The issue of gender is also worth considering in the context of our findings. Our sample contained more females than males, and the sexes were apportioned similarly across groups, although when their raw psychopathy scores were compared females obtained lower average scores (F = 295) than males (M = 322) (this difference was not statistically significant), supporting the representativeness of our male and female samples (Lilienfeld and Widows, 2005). We have some confidence that the results we observed were not confounded by sex differences in psychopathy, both because our groups were approximately evenly matched by sex and because preliminary evidence indicates that psychopathy may operate consistently across male and females (Miller et al., 2011). That said, it would be useful to more closely consider the issue of gender in future explorations, as previous investigations have found that in some contexts women show higher levels of empathy (Eisenberg and Lennon, 1983) and higher levels of fear responding than men, and it has been hypothesized that women’s increased fear responding may underlie their increased empathy and decreased aggression (Campbell, 2006). The present paradigm applied to larger male and female samples could provide a means for future testing to assess whether fear responding differences are linked to sex differences in psychopathy and/or patterns of neural activation.
Finally, the conclusions we can draw from our data are limited somewhat by the nature of the behavioral responses participants provided, which were binary yes/no responses. As a result, some participants provided no or almost no responses in one category or the other, limiting the utility of these responses as potential covariates in our imaging analyses. Future investigations could employ a wider range of response options. This would enable us to conduct more sensitive analyses to determine whether the patterns of neural responding we observed as a function of psychopathy drive the patterns of moral judgments we observed here and in previous research (Marsh and Cardinale, 2012). An alternate conclusion that could be drawn from the present data is that patterns of activation we observed, although they occur during moral judgments, reflect not the judgments themselves but simple responses to various emotion categories. The inclusion of conditions in which participants passively view the statements rather than making moral judgments might help to rule out this possibility.
CONCLUSIONS
Psychopathy has long been associated with two general domains of deficits: impoverished emotional responses, particularly to fear-relevant stimuli and increases in antisocial and aggressive behavior. Accumulating neuroscience research has identified brain regions in which dysfunction accompanies these traits, such as the amygdala. The results of this study link these features of psychopathy, showing that psychopathy is associated with reduced amygdala activity when judging the acceptability of frightening others and impaired judgments about the acceptability of causing others fear. These findings extend research on fear deficits and amygdala dysfunction in psychopathy to a new class of emotional stimuli and illuminate the putative relationship between emotional impairments and antisocial behavior in individuals with psychopathic traits. By linking psychopathic traits to reduced amygdala responses during judgments of verbal statements that elicit fear, the present study helps explain why psychopathy may increase the belief that to incite fear in another person is a morally acceptable course of action.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Leah Lozier, Molli Grossman, Caitlin Taylor and Justyna Mach for their assistance with data collection and analysis. This work was supported by NICHD grant R-03 HD064906 and by internal Georgetown University funding provided to A.A.M.
REFERENCES
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15:396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- Aniskiewicz AS. Autonomic components of vicarious conditioning and psychopathy. Journal of Clinical Psychology. 1979;35:60–7. doi: 10.1002/1097-4679(197901)35:1<60::aid-jclp2270350106>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65:1176–84. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–8. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DG, Richell RA, et al. Turning a deaf ear to fear: impaired recognition of vocal affect in psychopathic individuals. Journal of Abnormal Psychology. 2002;111:682–6. doi: 10.1037//0021-843x.111.4.682. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development & Psychopathology. 2005;17:865–91. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. Journal of Child Psychology & Psychiatry. 2005;46:327–36. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. Journal of Child Psychology & Psychiatry. 2006;47:262–76. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–19. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Campbell A. Sex differences in direct aggression: what are the psychological mediators? Aggression and Violent Behavior. 2006;11:237–64. [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity: An Attempt to Clarify Some Issues About the So-Called Psychopathic Personality. 5th edn. St. Louis, MO: Mosby; 1988. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, et al. Attention to eyes reverses fear recognition deficits in child psychopathy. British Journal of Psychiatry. 2006;189:280–1. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Dadds MR, El, Masry Y, Wimalaweera S, Guastella AJ. Reduced eye gaze explains ‘fear blindness’ in childhood psychopathic traits. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:455–63. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Fullam RS. Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent responses to emotional faces in violent patients with schizophrenia. Biol Psychiatry. 2009;66:570–7. doi: 10.1016/j.biopsych.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94:100–31. [Google Scholar]
- Farrow TF, Hunter MD, Wilkinson ID, et al. Quantifiable change in functional brain response to empathic and forgivability judgments with resolution of posttraumatic stress disorder. Psychiatry Research. 2005;140:45–53. doi: 10.1016/j.pscychresns.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168:152–62. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye—precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fullam RS, McKie S, Dolan MC. Psychopathic traits and deception: functional magnetic resonance imaging study. British Journal of Psychiatry. 2009;194:229–35. doi: 10.1192/bjp.bp.108.053199. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009a;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Young L, Hauser M. Increased DLPFC activity during moral decision-making in psychopathy. Molecular Psychiatry. 2009b;14:909–11. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Goldman AI, Sripada CS. Simulationist models of face-based emotion recognition. Cognition. 2005;94:193–213. doi: 10.1016/j.cognition.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–21. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RW, Mitchell DG. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Social Cognitive & Affect Neuroscience. 2012;7:958–68. doi: 10.1093/scan/nsr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. Toronto, Ontario: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD. Without Conscience: The Disturbing World of the Psychopaths Among Us. New York: Guilford; 1993. [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119:863–74. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kaufman AS. K-BIT: Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;15:6846–55. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular & Molecular Neurobiology. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive & Affect Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, Widows MR. PPI-R: Psychopathic Personality Inventory – Revised. Lutz, FL: Psychological Assessment Resources; 2005. [DOI] [PubMed] [Google Scholar]
- Lykken DT. The Antisocial Personalities. New York: Lawrence-Erlbaum; 1995. [Google Scholar]
- Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychological Bulletin. 2011;137:856–79. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger E, Mitchell DGV, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, et al. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research. 2011a;194:279–86. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2008;32:454–65. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Cardinale EM. Psychopathy and fear: specific impairments in judging behaviors that frighten others. Emotion. 2012;12:892–8. doi: 10.1037/a0026260. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EE, Schechter JC, Jurkowitz IT, Reid ME, Blair RJ. Adolescents with psychopathic traits report reductions in physiological responses to fear. Journal of Child Psychology & Psychiatry. 2011b;52:834–41. doi: 10.1111/j.1469-7610.2010.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. ; Proceedings of the National Academy of Sciences93; 1996. pp. 13508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Watts A, Jones SE. Does psychopathy manifest divergent relations with components of its nomological network depending on gender? Personality and Individual Differences. 2011;50:564–9. [Google Scholar]
- Munoz LC. Callous-unemotional traits are related to combined deficits in recognizing afraid faces and body poses. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:554–62. doi: 10.1097/CHI.0b013e31819c2419. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive Affective & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nichols S. Mindreading and the cognitive architecture underlying altruistic motivation. Mind & Language. 2001;16:425–55. [Google Scholar]
- Nunez JM, Casey BJ, Egner T, Hare T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. Neuroimage. 2005;25:267–77. doi: 10.1016/j.neuroimage.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Kavaliers M, Choleris E. Mechanisms underlying an ability to behave ethically. American Journal of Bioethetics. 2008;8:10–9. doi: 10.1080/15265160802179994. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Poythress NG, Lilienfeld SO, Skeem JL, et al. Using the PCL-R to help estimate the validity of two self-report measures of psychopathy with offenders. Assessment. 2010;17:206–19. doi: 10.1177/1073191109351715. [DOI] [PubMed] [Google Scholar]
- Raine A, Dodge K, Loeber R, et al. The reactive–proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior. 2006;32:159–71. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61:1260–71. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Scott SK, Young AW, Calder AJ, et al. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;385:254–7. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behavioral Sciences & the Law. 2009;27:137–71. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeem JL, Cooke DJ. Is criminal behavior a central component of psychopathy? Conceptual directions for resolving the debate. Psychological Assessment. 2010;22:433–45. doi: 10.1037/a0008512. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Polaschek DLL, Patrick CJ, Lilienfeld SO. Psychopathic personality: Bridging the gap between scientific evidence and public policy. Psychological Science in the Public Interest. 2011;12:95–162. doi: 10.1177/1529100611426706. [DOI] [PubMed] [Google Scholar]
- Sylvers PD, Brennan PA, Lilienfeld SO. Psychopathic traits and preattentive threat processing in children: a novel test of the fearlessness hypothesis. Psychological Science. 2011;22:1280–7. doi: 10.1177/0956797611420730. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behavioral & Brain Sciences. 1990;13:109–20. [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Widom CS. A methodology for studying noninstitutionalized psychopaths. Journal of Consulting & Clinical Psychology. 1977;45:674–83. [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–94. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]