Abstract
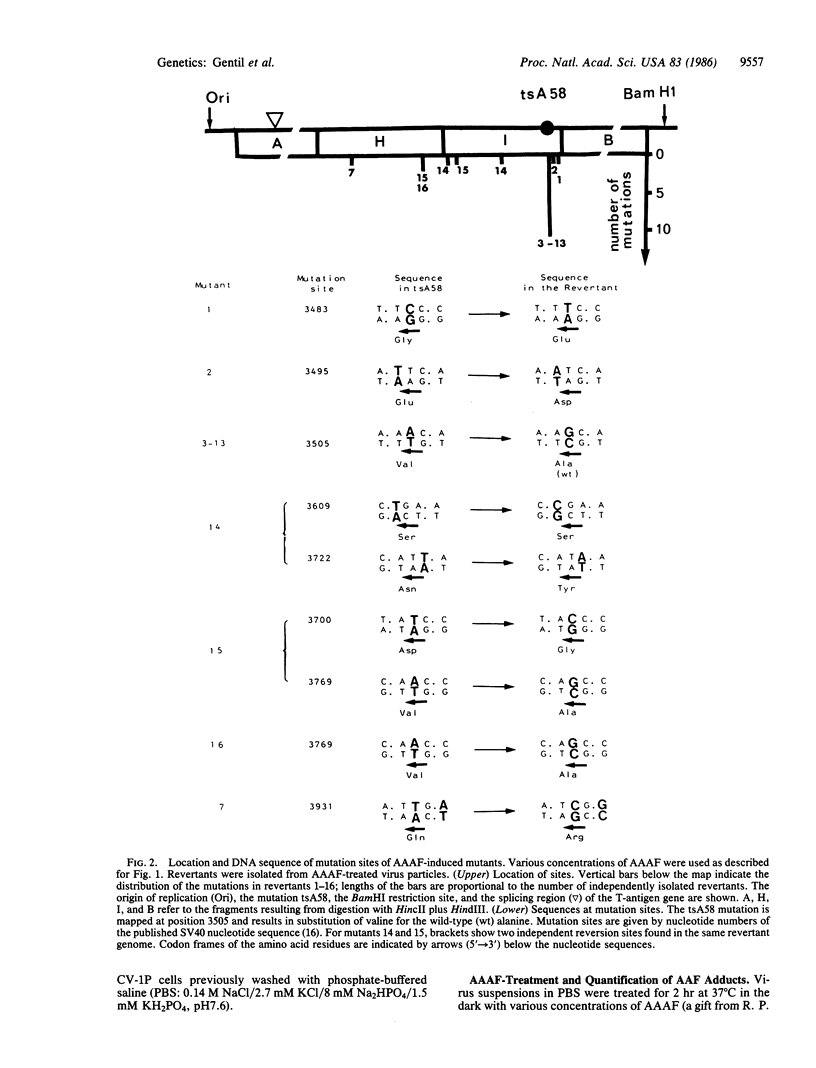
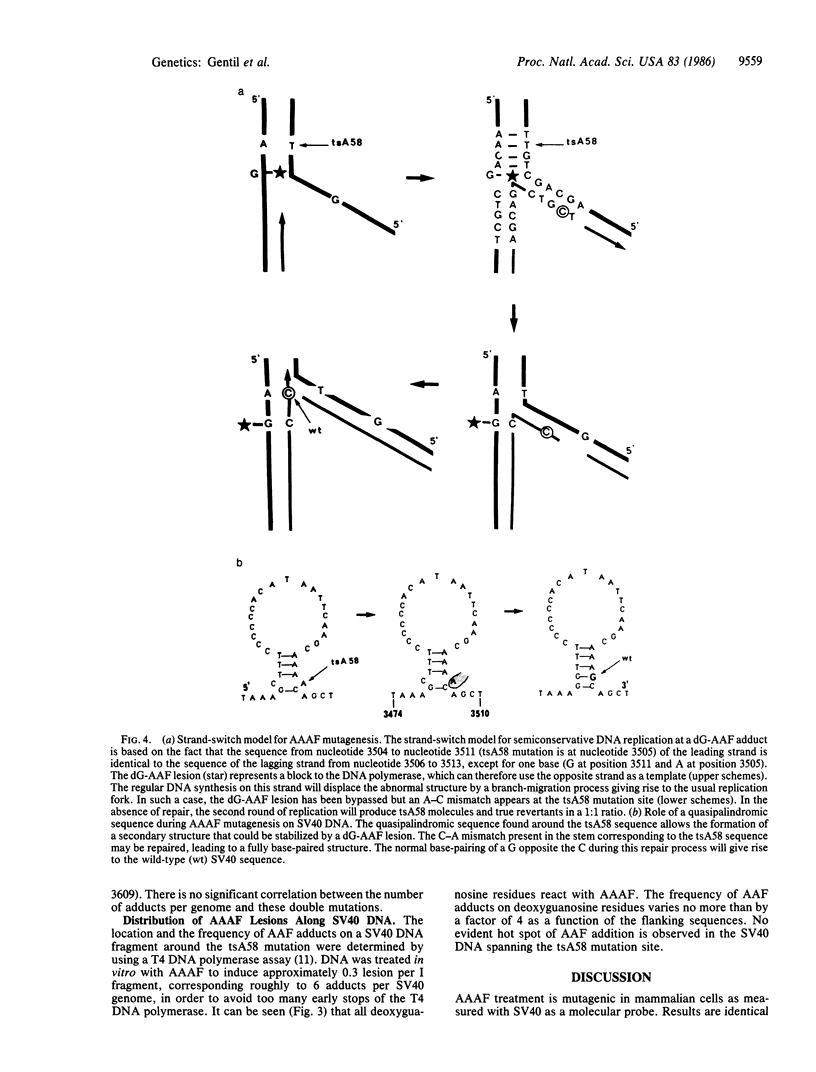
Mutations induced by 2-(N-acetoxy-N-acetylamino)fluorene were studied using temperature-sensitive simian virus 40 (SV40) mutants as probe in monkey kidney cells. In vitro treatment of the SV40 virions with 2-(N-acetoxy-N-acetylamino)fluorene increased mutagenesis and decreased survival in the viral progeny. A lethal hit of approximately 85 acetylaminofluorene adducts per SV40 genome was calculated. UV irradiation of cells prior to infection did not modify the results. Molecular analysis of independent SV40 revertants showed that 2-(N-acetoxy-N-acetylamino)fluorene induces base substitutions that are located not opposite putative acetylaminofluorene adducts but next to them. Moreover, a hot spot of mutation restoring a true wild-type genotype was observed in 10 of the 16 revertants analyzed. This hot spot, not targeted opposite a major DNA lesion, was not observed using UV light as damaging agent in the same genetic assay. Two models involving the stabilization, by acetylaminofluorene adducts, of the secondary structure of a specific quasipalindromic SV40 sequence are proposed to explain this sequence-specific hot spot.
Full text
PDF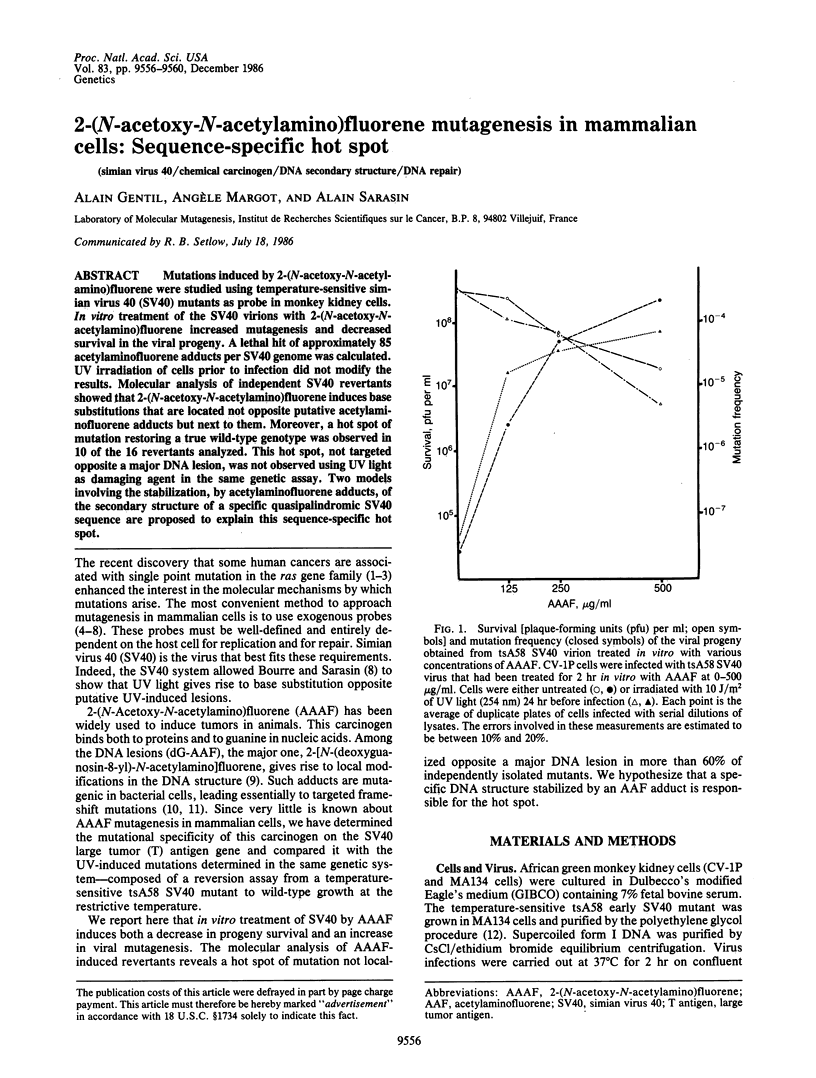


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourre F., Sarasin A. Targeted mutagenesis of SV40 DNA induced by UV light. Nature. 1983 Sep 1;305(5929):68–70. doi: 10.1038/305068a0. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Lefevre J. F., Pouyet J., Daune M. P. Comparative orientation of the fluorene residue in native DNA modified by N-acetoxy-N-2-acetylaminofluorene and two 7-halogeno derivatives. Biochemistry. 1976 Jul 27;15(15):3347–3351. doi: 10.1021/bi00660a027. [DOI] [PubMed] [Google Scholar]
- Fujita J., Yoshida O., Yuasa Y., Rhim J. S., Hatanaka M., Aaronson S. A. Ha-ras oncogenes are activated by somatic alterations in human urinary tract tumours. 1984 May 31-Jun 6Nature. 309(5967):464–466. doi: 10.1038/309464a0. [DOI] [PubMed] [Google Scholar]
- Gentil A., Margot A., Sarasin A. Apurinic sites cause mutations in simian virus 40. Mutat Res. 1984 Nov;129(2):141–147. doi: 10.1016/0027-5107(84)90146-5. [DOI] [PubMed] [Google Scholar]
- Golding G. B., Glickman B. W. Sequence-directed mutagenesis: evidence from a phylogenetic history of human alpha-interferon genes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8577–8581. doi: 10.1073/pnas.82.24.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Ripley L. S. Model for the participation of quasi-palindromic DNA sequences in frameshift mutation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4128–4132. doi: 10.1073/pnas.79.13.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Induction of an error-prone mode of DNA repair in UV-irradiated monkey kidney cells. Mutat Res. 1980 Mar;70(1):71–81. doi: 10.1016/0027-5107(80)90059-7. [DOI] [PubMed] [Google Scholar]
- Sarasin A., Bourre F., Benoit A. Error-prone replication of ultraviolet-irradiated simian virus 40 in carcinogen-treated monkey kidney cells. Biochimie. 1982 Aug-Sep;64(8-9):815–821. doi: 10.1016/s0300-9084(82)80135-1. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. A., Glickman B. W. Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4123–4127. doi: 10.1073/pnas.79.13.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. G., Ripley L. S. Demonstration of the production of frameshift and base-substitution mutations by quasipalindromic DNA sequences. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5528–5531. doi: 10.1073/pnas.81.17.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]


