In our Monte Carlo model comparing management strategies for recurrent urinary tract infections in women, we found that nitrofurantoin prophylaxis was most effective, but most expensive to the payer. Other strategies resulted in payer savings but were less efficacious.
Keywords: urinary tract infection, recurrent, management
Abstract
Background. Recurrent urinary tract infections (UTIs) are a common problem among women. However, comparative effectiveness strategies for managing recurrent UTIs are lacking.
Methods. We performed a systematic literature review of management of women experiencing ≥3 UTIs per year. We then developed a Markov chain Monte Carlo model of recurrent UTI for each management strategy with ≥2 adequate trials published. We simulated a cohort that experienced 3 UTIs/year and a secondary cohort that experienced 8 UTIs/year. Model outcomes were treatment efficacy, patient and payer cost, and health-related quality of life.
Results. Five strategies had ≥2 clinical trials published: (1) daily antibiotic (nitrofurantoin) prophylaxis; (2) daily estrogen prophylaxis; (3) daily cranberry prophylaxis; (4) acupuncture prophylaxis; and (5) symptomatic self-treatment. In the 3 UTIs/year model, nitrofurantoin prophylaxis was most effective, reducing the UTI rate to 0.4 UTIs/year, and the most expensive to the payer ($821/year). All other strategies resulted in payer cost savings but were less efficacious. Symptomatic self-treatment was the only strategy that resulted in patient cost savings, and was the most favorable strategy in term of cost per quality-adjusted life-year (QALY) gained.
Conclusions. Daily antibiotic use is the most effective strategy for recurrent UTI prevention compared to daily cranberry pills, daily estrogen therapy, and acupuncture. Cost savings to payers and patients were seen for most regimens, and improvement in QALYs were seen with all. Our findings provide clinically meaningful data to guide the physician–patient partnership in determining a preferred method of prevention for this common clinical problem.
(See the Editorial Commentary by Gupta and Bhadelia on pages 161–3.)
Urinary tract infections (UTIs) are a common problem among women. It has been estimated that 10%–13% of women experience a UTI annually with a lifetime risk of UTI of >50% [1, 2]. In the United States, UTIs result in >6 million outpatient visits [3] and 479 000 hospitalizations annually [4]. The associated annual societal cost of UTI treatment has been estimated to exceed $2.47 billion annually [2]. UTIs also impact quality of life. Women suffering from UTIs report pain, restriction of work and school, and bed rest as a result of these infections [5]
Recurrent UTIs are a common problem seen in clinical practice [6–8]. After an initial UTI, approximately 20%–30% of women with a UTI will have a second UTI within 6 months and 3% will experience a third UTI during that time period [6]. There are several strategies used for the management of recurrent UTIs that include antibiotic prophylaxis, acupuncture, estrogens, and cranberry products [9–25]. Additionally, several studies have examined symptomatic self-treatment, that is, having a woman self-diagnose a UTI and subsequently self-treat [26–28].
It is unclear which strategies are optimal to manage women with recurrent UTIs. There have been no data syntheses of management strategies. To address the comparative effectiveness of managing recurrent UTIs in women, we performed a decision analysis comparing the effectiveness, cost, and health-related quality-of-life (HRQOL) outcomes associated with commonly used strategies for management of recurrent UTIs.
METHODS
For our investigation, we used a Markov decision analysis to evaluate the effectiveness of 5 strategies to prevent recurrent UTIs. We chose only strategies in which there were ≥2 clinical trials in the published literature. For each strategy, we used a Monte Carlo simulation of a cohort of subjects.
We measured several outcomes for each strategy: (1) number of UTIs per year; (2) annual cost from the payer's (ie, health plan's) perspective; (3) annual cost from the patient's perspective; and (4) quality-adjusted life-days (QALDs). This last metric was used instead of the traditional quality-adjusted life-years (QALYs), as the Markov model's unit measure of time was 1 day. A QALD was defined as a QALY divided by 365. We also measured cost-effectiveness, defined as the cost per QALY gained [29]. The decision analysis model was conducted using DATA version 4.0 (TreeAge Software, Williamstown, Massachusetts).
Systematic Literature Review
To obtain information on clinical outcomes and cost of prevention of recurrent UTIs, a systematic review of the literature was performed. Medline was searched for articles from 1966 to January 2012 with the following keywords: (1) recurrent [recur*], (2) urine or urinary [urin*], and (3) infectious or infection(s) [infectious, infection*]. The search was limited to the English language and human research. Identical systematic searches were conducted using Embase and Cochrane Library databases.
Two reviewers (from among K.B., S.J.E., J.A.M., and L.G.M.) assessed each abstract. If both reviewers believed an abstract might contain data on the recurrent UTI management or was a review article that may reference such data, the article was pulled for review. If the reviewers differed in assessment, a third reviewer served as a tiebreaker. Reference lists of retrieved articles were also reviewed to find additional studies. The inclusion criteria for an article to be reviewed included all of the following: (1) The study population was comprised of adult nonpregnant female subjects; (2) the study population had ≥3 UTIs per year; and (3) the study was a comparative clinical trial using either an untreated control group or a preintervention and postintervention comparison of UTI incidence.
UTI incidence reduction was obtained only from articles presenting original data not published elsewhere. From each article, the risk reduction was calculated by comparing the prevention strategy to the nontreated or the preintervention UTI group. A mean risk reduction weighted by study sample size was calculated for each strategy.
Markov Model
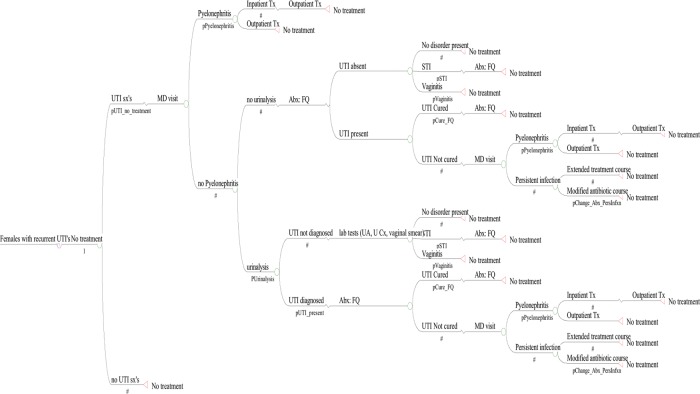
Our Markov model assumed that each day, a patient is always in 1 health state (Figure 1). A Monte Carlo evaluation of this Markov model was used to determine the outcomes of 10 000 independent persons undergoing each intervention and a sixth cohort that underwent no intervention. During each day of the Markov cycle, a patient may transition from one state to another, as determined by transition probabilities. Each simulation generated a cumulative cost and a QALD score for each individual. From each cohort, mean costs, cost-effectiveness, and QALD were calculated.
Figure 1.
Markov decision tree of urinary tract infection (UTI) outcomes among patients with recurrent UTIs. Abbreviations: Abx, antibiotic; FQ, fluoroquinolone; MD, physician; STI, sexually transmitted infection; Tx, treatment; UA, urinalysis; U Cx, urine culture; UTI, urinary tract infection.
Model Structure
The model assumed that each cohort was participating in each strategy for 1 year and that patients in the no intervention strategy cohort had a daily UTI risk that resulted in a mean of 3 UTIs/year. We also performed a secondary model that assumed that persons suffered from a higher UTI rate (8 UTIs/year). UTI risk reduction was calculated as [daily risk of UTIs if untreated] × [risk reduction of prevention strategy].
The model assumed that each UTI would ultimately resolve in cure after antibiotic treatment (Figure 1). In the model, if during the day (Markov cycle period), the woman did not develop UTI symptoms, she would not receive any treatment (Figure 1). If on a given day a patient developed UTI symptoms, the model assumed the patient would visit a clinician in the outpatient setting (unless she was in the symptomatic self-treatment cohort). The events that would occur upon presentation to care for UTI symptoms were based upon previous decision analysis models of acute UTIs [30–32]. A proportion of women with UTI symptoms would have cystitis whereas others would present with pyelonephritis [33, 34]; a proportion of those in the latter group would require hospitalization [35–37] Those not hospitalized for pyelonephritis were treated on an outpatient basis with 10 days of an oral fluoroquinolone [33, 34, 38, 39].
Upon outpatient presentation for cystitis, the patient was diagnosed with a UTI and given a prescription for ciprofloxacin for 7 days. A proportion of patients would have urinalysis sent by their physician [40] (Figure 1). For patients with UTI symptoms and a urinalysis performed, there was a probability of having a UTI, sexually transmitted infection (STI), or vaginitis [41, 42], and a proportion of patients who did not actually have a UTI were instead diagnosed with one of the latter 2 diagnoses [41, 42]. We modeled a common complication of treatment, specifically candidal vaginitis [41, 42]. A proportion of patients with a UTI present would be cured with the initial antibiotic course. The remaining women would have an uncured UTI and would return to the outpatient clinic resulting in a diagnosis of either pyelonephritis or persistent infection. Patients with a persistent infection were prescribed a prolonged course of antibiotics or received intramuscular ceftriaxone or gentamicin [43–48].
Cost Analyses
We performed cost analyses from the program perspective and the patient perspective. Our model assumed that treatment costs were borne by the healthcare payer (ie, insurance company) except for copays. The model assumed that the patient bore the cost of cranberry pills and acupuncture treatment.
Hospitalization costs were derived from a national survey and incorporated the mean daily cost of hospitalization from the American Hospital Association [49]. Costs of physician visits were derived from the literature and from physician payment schedules available from the Centers for Medicare and Medicaid Services [50–53]. For the cost of outpatient pyelonephritis treatment, it was assumed that ciprofloxacin 500 mg twice daily was used as therapy. Costs of urinalysis, urine culture, vaginal smear, and STI testing were calculated as an average cost from ≥3 major commercial and state laboratories [32]. The cost of self-treatment for vaginal yeast infection was calculated from a survey of prices for over-the-counter antifungal products available from 3 major national pharmacy stores [32]. All costs were calculated in 2010 US dollars.
Annual cost from the patient perspective was determined by choosing the mean cost to the cohort for each intervention. The model assumed that each physician visit required a $25 copay, and that medications required a $10 copay per prescription. Daily cranberry pill cost was calculated based on a survey of all available cranberry products offered by 8 major national pharmacy stores. The cost of monthly acupuncture sessions was determined by calculating the average cost of a session plus cost of the initial session from 30 different acupuncture clinics across the United States. For the hospitalization cost, the model assumed that the patient incurred 10% of the total cost. All other costs, including those of laboratory or diagnostic testing, were assumed to be covered by the patient's insurance.
Health-Related Quality of Life
QALDs were estimated for each patient using clinically meaningful outcomes experienced by a patient (such as days affected by UTI symptoms, restricted activity, bed days, or days of acupuncture treatment). The values were obtained from the literature [54]. The outcomes of both QALY saved and cost per QALY saved were calculated for each strategy and compared to the no intervention strategy.
Sensitivity Analyses
One-way sensitivity analyses were performed for each cost, probability, and QALD value to determine the variables influential on the outcomes of interest. Each value was varied over the minimal and maximal values determined from the literature or our surveys of cost. If no minimal and/or maximal value existed, we varied the range from 50% to 200% of the value in the model.
RESULTS
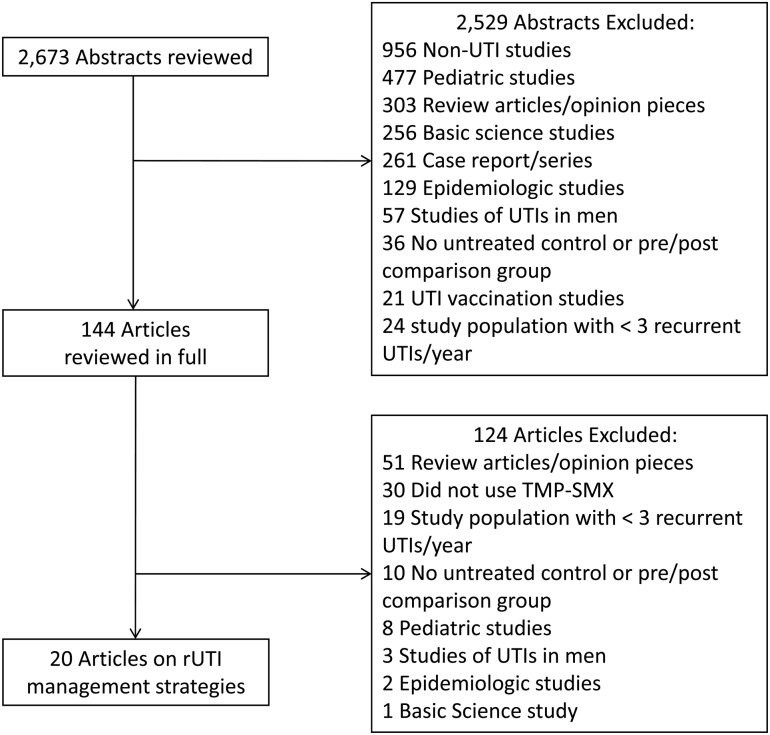
The systematic review of the literature yielded 2673 articles, from which we found 20 articles that were clinical trials of recurrent UTI management that met our criteria, 2 using acupuncture prophylaxis, 4 using cranberry prophylaxis, 5 using estrogen prophylaxis, 6 using antibiotic (nitrofurantoin, the most commonly studied agent) prophylaxis, and 3 using self-treatment) (Figure 2). Probabilities and costs and the associated ranges are summarized in Tables 1 and 2, respectively.
Figure 2.
Study selection process and reasons for exclusion of references. In cases where articles fell into >1 category, the primary reason for exclusion is noted. Abbreviations: rUTI, recurrent urinary tract infection; TMP-SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
Table 1.
Probability Values for Variables in Model
| Description | Probability | Range of Probabilities Tested | References |
|---|---|---|---|
| Acupuncture risk reduction | 0.68 | 0.6–0.7 | [24, 25] |
| Cranberry risk reduction | 0.50 | 0.4–0.8 | [9, 10, 16, 17] |
| Daily antibiotics/nitrofurantoin, 100 mg once daily risk reduction | 0.86 | 0.6–1.0 | [11, 19–23] |
| Estrogen use risk reduction | 0.65 | 0.3–1.0 | [12–15, 18] |
| Clinical cure of fluoroquinolone-sensitive infection treated with fluoroquinolone | 0.94 | 0.9–1.0 | [46, 55–57] |
| Vaginal yeast infection after ≤3 d of therapy | 0.05 | 0–0.2 | [34, 57, 58] |
| Vaginal yeast infection after >3 d of therapy | 0.07 | 0–0.2 | [46, 59, 60] |
| Medical visit for vaginal yeast infection | 0.25 | 0–0.5 | [53] |
| Change of therapy due to lack of clinical response (vs extending treatment) | 0.75 | 0–1 | [33, 34] |
| Physician orders urinalysis | 0.769 | 0.25–1 | [40] |
| UTI when symptoms are present | 0.8481 | 0.6–1 | [26–28] |
| Pyelonephritis | 0.04 | 0.00–0.08 | [33, 34, 38, 39] |
| Outpatient treatment for pyelonephritis | 0.80 | 0.5–1 | [33, 34] |
| STI present | 0.157 | 0–0.5 | [41] |
| Vaginitis present | 0.133 | 0–0.5 | [41, 42] |
| No disorder present | 0.709 | 0.5–1 | [42] |
Abbreviations: STI, sexually transmitted infection; UTI, urinary tract infection.
Table 2.
Cost of Interventions to Prevent or Treat Recurrent Urinary Tract Infections and Duration of Clinical Events
| Description | Mean Cost per Unit Time or per Item (US$) | Range Tested (US$) | References |
|---|---|---|---|
| Acupuncture, initial session fee plus each monthly sessiona | 2.51/d | 1.37–4.60 | |
| Cranberry pillb | 0.75/d | 0.13–2.25 | |
| Estrogen | 0.50/d | 0.14–31.63 | [61] |
| Daily antibiotics/nitrofurantoin, 100 mg once daily (AWP) | 1.95/d | 1–4 | [61] |
| Ciprofloxacin, 250 mg twice daily (AWP) | 4.44/d | 2–10 | [61] |
| Ciprofloxacin, 500 mg twice daily (AWP) | 5.38/d | 2–11 | [61] |
| Self-treatment for yeast infection | 16.14 | 8–32 | [32] |
| Hospitalization for pyelonephritis | 1782.28/d | 850–3600 | [49] |
| Outpatient treatment for infection unresponsive to fluoroquinolones or pyelonephritis | 29.77/d | 15–60 | [61] |
| Follow-up physician visit | 97.77 | 65–132 | [50–53] |
| Initial urinalysis | 20.78 | 10–42 | [32] |
| Follow-up urinalysis | 20.78 | 10–42 | [32] |
| Urine culture | 46.42 | 23–93 | [32] |
| Vaginal smear | 13.50 | 10.65–16.25 | [32] |
| STI test | 67.60 | 20–155 | [32] |
| Description, duration, d (range) | |||
| Hospitalization for pyelonephritis | 3 | 1–5 | [37, 38] |
| Outpatient treatment for infection unresponsive to fluoroquinolones | 5 | 3–10 | [38] |
| Outpatient treatment for pyelonephritis | 7 | 5–14 | [55, 62] |
Abbreviations: AWP, average wholesale price; STI, sexually transmitted infection.
a Cost of monthly acupuncture sessions was determined by calculating the average cost of a session plus cost of the initial session from 30 different acupuncture clinics across the United States: Acupuncture & Chinese Herbs (Virginia Beach, Virginia), Acupuncture and Holistic Health Center (Jacksonville, Florida), Acupuncture Berea (Berea, Kentucky), Acupuncture Center (Maui, Hawaii), Akimi Acupuncture (Carmel, Indiana), Alchemy Healing Arts (Mill Creek, Washington), Berkeley Community Acupuncture (Berkeley, California), Boulder Holistic Acupuncture (Boulder, Colorado), Charlotte Acupuncture & Wellness Center (Charlotte, North Carolina), Cumming Acupuncture & Chinese Herbal Medicine (Cumming, Georgia), Dakota Family Acupuncture (Bismarck, North Dakota), East Mountain Acupuncture (Cold Spring, New York), Edgewater Athletic Club (Chicago, Illinois), Goforth Acupuncture and Massage (Solana Beach, California), Healing Hands Chiropractic Family Wellness Center (Londonderry, New Hampshire), Healing Tao Institute (Austin, Texas), Hobbs Acupuncture and Integrated Medicine (Atlanta, Georgia), Holistic Points (Hudson, Ohio), Intouch (New York City, New York), Live Oak Acupuncture & Herbal Medicine (Waco, Texas), Minneapolis Acupuncture (Minneapolis, Minnesota), Northwoods Acupuncture and Massage (Woodruff, Wisconsin), Pathways to Wellness (Boston, Massachusetts), Peaceful Spirit Massage & Wellness Centers (Tucson, Arizona), Seattle Athlete Acupuncture Clinic (Seattle, Washington), Spokane Acupuncture Clinic (Spokane, Washington), Sunrise Acupuncture Clinic (Salt Lake City, Utah), Triplett Acupuncture (Oklahoma City, Oklahoma), Wilson Acupuncture & Healing Arts Center (Wilson, Wyoming), Yo San Community Clinic (Los Angeles, California).
b Daily cranberry pill cost was calculated based on a survey of all available cranberry pill products offered by 8 major national pharmacy stores (Amazon, CVS, drugstore.com, GNC, Rite Aid, The Vitamin Shoppe, Walgreens, and Walmart).
Payer Perspective Model
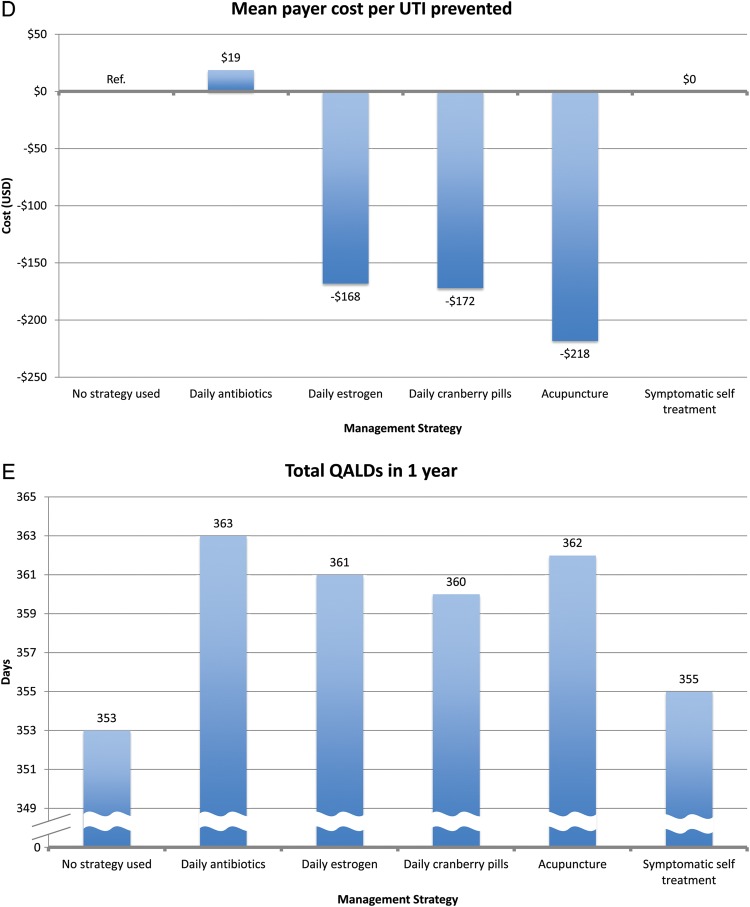
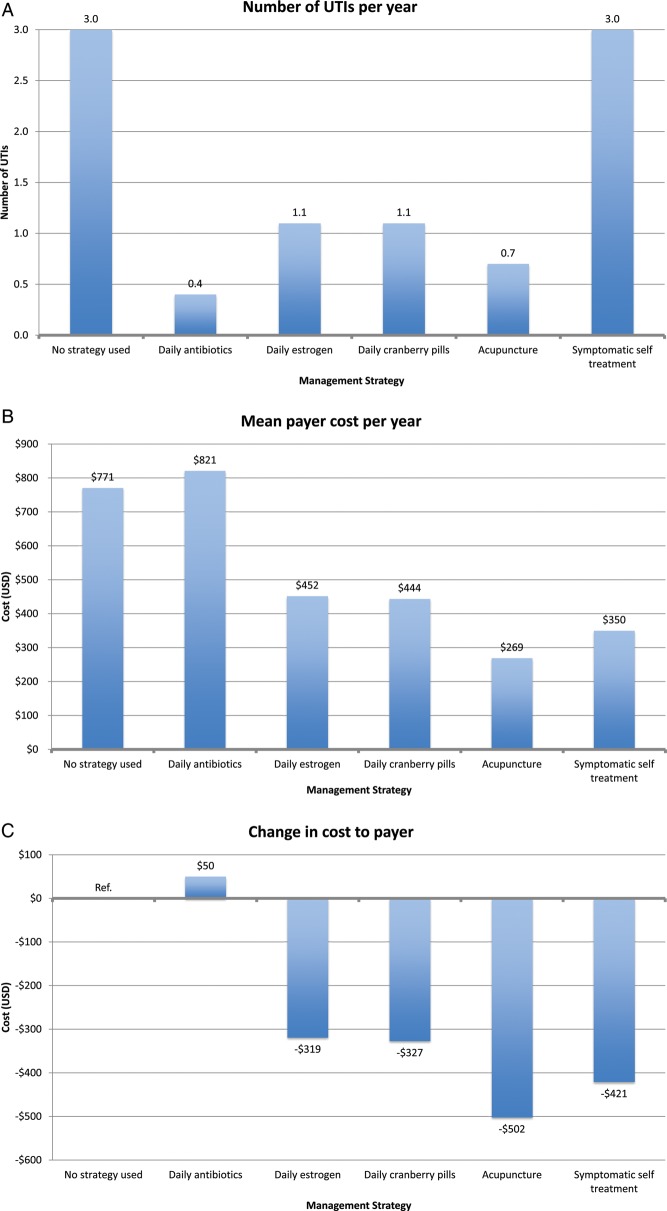
In our payer perspective model, patients in the no intervention cohort experienced 3.0 UTIs/year with a mean annual payer cost of $771 (Figure 3). All prevention strategies resulted in a reduction in UTI rate. Daily antibiotic prophylaxis was most effective at UTI reduction, with a UTI rate of 0.4/year. However, daily antibiotic prophylaxis was the most expensive intervention, with a mean annual payer cost of $821. Symptomatic self-treatment did not reduce the number of UTIs/year, which remained at 3.0 UTIs/year, but the mean annual payer cost was $350.
Figure 3 continued.
Figure 3 continued.
Figure 3.
A–G, Results of decision analysis of management strategies for recurrent urinary tract infections (UTIs) from the payer perspective among women experiencing 3 UTIs per year. Abbreviations: QALD, quality-adjusted life-day; QALY, quality-adjusted life-year; Ref., reference group; USD, US dollars; UTI, urinary tract infection.
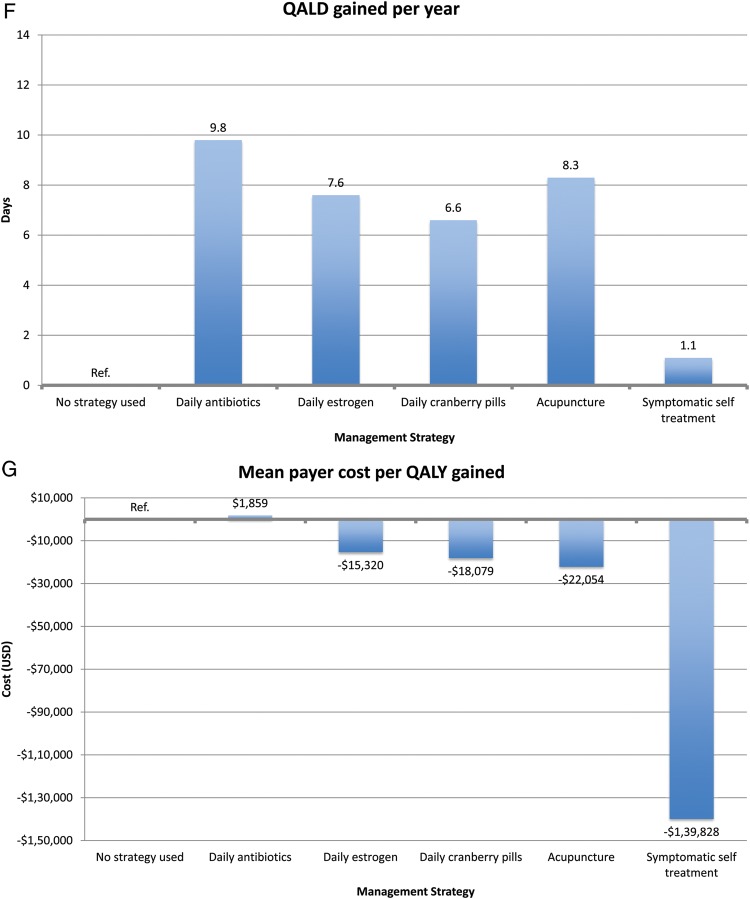
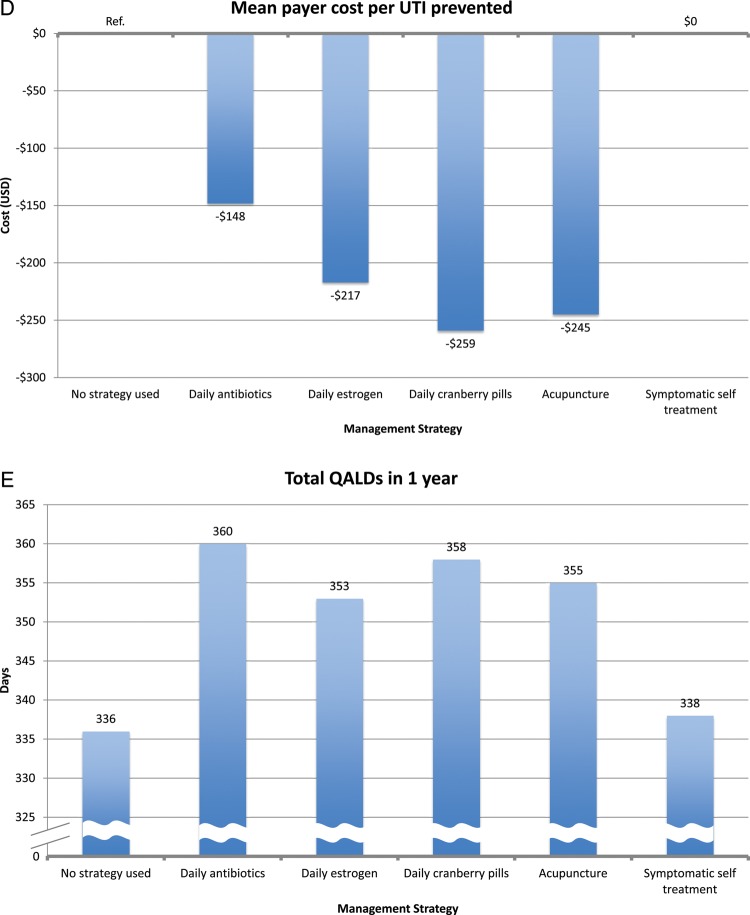
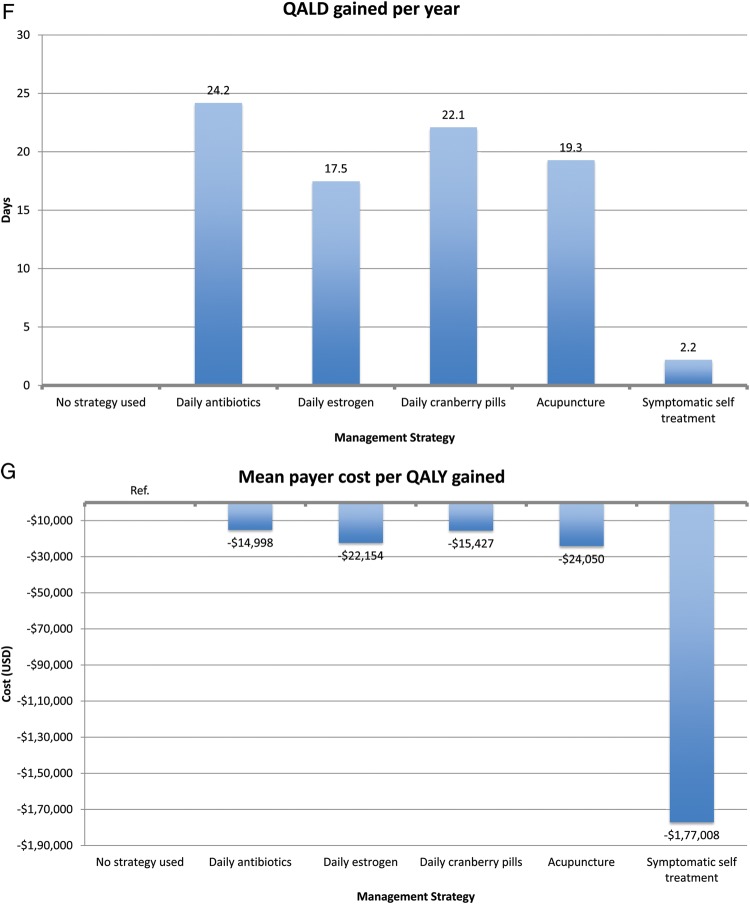
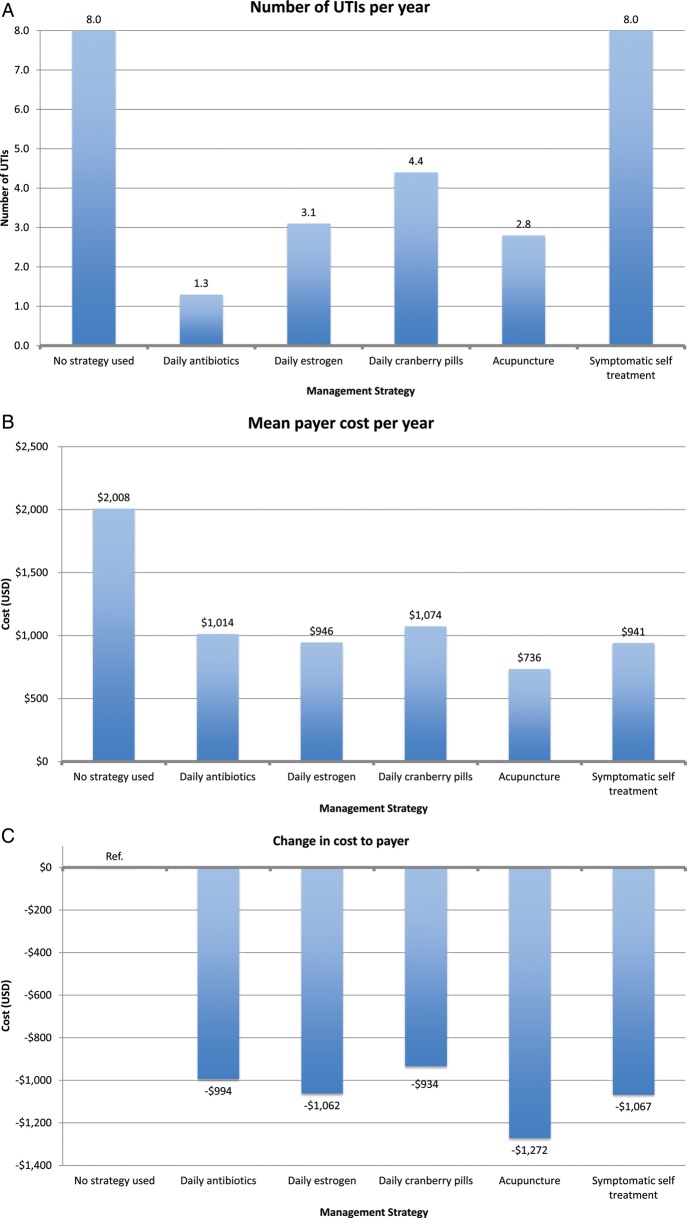
All other prophylaxis strategies reduced UTI rates, ranging from 0.7 UTIs/year for acupuncture to 1.1 UTIs/year for estrogen and cranberry prophylaxis (Figure 3). All other prophylaxis strategies resulted in a payer cost savings, ranging from $319/year for estrogen prophylaxis to $502/year for acupuncture prophylaxis. All strategies resulted in a cost savings per QALY gained for the payer except daily antibiotic prophylaxis ($1859 per QALY gained). In the model of patients experiencing 8 UTIs/year, we found similar results except that daily antibiotic prophylaxis now resulted in payer cost savings (Figure 4).
Figure 4 continued.
Figure 4 continued.
Figure 4.
A–G, Results of decision analysis of management strategies for recurrent urinary tract infections (UTIs) from the payer perspective among women experiencing 8 UTIs per year. Abbreviations: QALD, quality-adjusted life-day; QALY, quality-adjusted life-year; Ref., reference group; USD, US dollars; UTI, urinary tract infection.
Patient Perspective Model
From the patient perspective, symptomatic self-treatment was the only cost-saving strategy, with a mean savings of $70/year (Figure 3; also see online supplementary material). All prophylactic interventions incurred additional costs to the patient, ranging from the least expensive (daily antibiotics), mean of $140/year, to the most expensive (acupuncture), mean of $946/year. In contrast, patients in the no intervention strategy incurred a mean annual out-of-pocket cost of $139.
In terms of cost per QALY gained, self-treatment was the most effective (cost savings of $23 260 per QALY gained). Other interventions incurred cost per QALY gained. The most cost-effective strategy was daily antibiotic prophylaxis ($19 per QALY gained), and the least cost-effective strategy was acupuncture ($35 467 per QALY gained). In the 8 UTI/year model, we had similar findings. However, symptomatic self-treatment, antibiotic prophylaxis, and estrogen prophylaxis all resulted in cost savings to the patient.
Sensitivity Analyses
In our sensitivity analysis, for the payer models, change in daily estrogen cost was the most influential cost variable, resulting in a 30% reduction in overall cost if estrogen cost was $0.14/day or a 2400% increase if the cost was $32/day. Daily antibiotic prophylaxis cost was the next most influential cost variable with a range from a 43% reduction to an 89% increase in overall cost to the prophylaxis strategy. Other costs had lesser effects.
In the patient perspective models, daily cranberry prophylaxis cost was the most influential cost variable, resulting in a change from a 64% reduction to a 155% increase in overall patient cost. The costs of acupuncture prophylaxis was the next most influential cost variable to the patient, resulting in change from a 43% reduction to a 79% increase in annual patient cost.
In our sensitivity analyses, we also found that the probability of a UTI occurring was very influential in determining the number of UTIs experienced per year for each prophylaxis strategy. Sensitivity analysis for daily antibiotic prophylaxis resulted in the largest range of UTIs experienced per year of all of the strategies. The range used for daily antibiotic prophylaxis risk reduction was 0.6–1.0, which resulted in a UTI rate ranging from 0.0 to 1.2 UTIs per year. This large range likely reflects the relatively large amount of nitrofurantoin prophylaxis studies in the literature. The probability of pyelonephritis was the most influential probability on overall payer cost, ranging from a 23% reduction to a 346% increase of overall payer cost as the probability in the model ranged from 0.0 to 0.8. To a lesser extent, pyelonephritis probability affected patient costs (5% reduction to a 191% increase in overall costs). The acute UTI cure rate using a fluoroquinolone was the variable most influential on the QALD, with a range of 348–361 QALDs when the cure rate ranged from 90% to 100%.
DISCUSSION
Using a decision analysis model, we examined the comparative effectiveness of 5 management strategies for recurrent UTIs. Among our models of women experiencing 3 UTIs and 8 UTIs per year, all 4 prevention strategies—daily antibiotics, daily estrogen, daily cranberry pills, and monthly acupuncture sessions—resulted in a lower UTI rate. All strategies are also well under the National Institute for Health and Care Excellence (UK) threshold for cost-effectiveness [29].
Daily antibiotic prophylaxis is the most extensively studied prevention strategy for recurrent UTIs [11, 19–23]. This strategy was most effective at reducing UTI incidence and was one of the least expensive strategies for the patient. Antibiotic prophylaxis also resulted in cost savings per QALY gained in both models. Thus, antibiotic prophylaxis may provide a reasonable strategy for both payer and patient. It should be noted that the studies of antibiotic UTI prophylaxis examined daily nitrofurantoin (100 mg) use. The benefits of other antibiotics or other dosing regimens are not as well studied.
Somewhat surprisingly, we found that acupuncture was the next most effective prevention method. Acupuncture's high efficacy may be a function of publication bias, as there were fewer studies on acupuncture compared to other management strategies. Although acupuncture prophylaxis is the least expensive strategy from the payer prospective, the cost is borne by the patient, as insurers do typically not cover acupuncture. Acupuncture may not appeal to some patients, and some may have challenges to access this treatment modality.
Daily estrogen use and daily cranberry pills resulted in similar reductions in UTI rate and payer cost per QALY gained. However, payers rarely cover cranberry cost, placing the financial burden on the patient. Additionally, a standardized dosage of cranberry pills to prevent recurrent UTIs is poorly defined [63]. Of note, 2 recent clinical trials found that cranberry prophylaxis was ineffective at reducing subsequent UTIs [64, 65]. However, in both investigations, the study populations were not at high risk for recurrent UTI and had a UTI rate far below that of our prespecified criteria. Additionally, a recent investigation compared cranberry juice to trimethoprim-sulfamethoxazole antibiotic prophylaxis [66]. However, we did not include this study in our review, as there was no placebo or comparison with no treatment. Estrogen prophylaxis is limited to postmenopausal women [67]. The optimal delivery method (transdermal, oral, or topical) for UTI prophylaxis is unclear [67, 68].
We found unique merits to symptomatic self-treatment. This strategy is the most cost-minimizing strategy to both payer and patient. The savings was largely due to decreased physician visits and hospitalizations. However, symptomatic self-treatment does not result in a lower UTI rate and had only modest QALY increases (1.1 QALD in the 3 UTIs model and 2.2 QALDs in the 8 UTIs model). We assume that some women enduring recurrent UTIs may prefer to reduce UTI incidence and may not prefer this strategy.
Our investigation has limitations. The risk reduction values for each strategy are based on published studies. Publication bias may result in overestimates of efficacy. Additional factors such as infection with multidrug-resistant organisms, medication adherence, long-term tolerability, toxicity, and uncommon adverse reactions are not explicitly accounted for in our model. However, in our sensitivity analyses, expensive but rare events had minimal effect on overall cost [30, 32]. Our investigation is also limited by use of generic HRQOL measures [54]. However, disease-specific HRQOL for patients with UTIs do not exist. We did also not examine Lactobacillus as a preventive strategy, as at the time of our investigation there was only 1 investigation that fit our predefined inclusion criteria [69]. A recently published trial comparing proprietary Lactobacillus strains to trimethoprim-sulfamethoxazole suggests that efficacy was similar in the 2 treatment groups [70]. Depending on the cost, this strategy may have similar advantages and disadvantages to cranberry and acupuncture interventions. We did not examine recurrent UTI management strategies in women with less common rates of recurrent UTIs (ie, <3 UTIs/year). It is unknown with currently available data if these management strategies would be beneficial among this population. We also found that our model's findings could be influenced by several variables such as chance of pyelonephritis when a UTI occurs. These variables identified in sensitivity analysis should be the target of further studies. Finally, comparative clinical trials (eg, placebo-controlled trials) are the ideal method for determining efficacy, and some of the trials we used to determine probabilities had methodological limitations (ie, before–after designs).
There are strengths to our investigation. First, our decision analysis utilized multiple and complementary outcomes: efficacy, HRQOL, and cost from the patient and payer perspectives. A second strength is that the data used in our model are based on a systematic literature review. Finally, our investigation provides summary data enabling clinicians and patients to compare strategies and choose a management strategy that most suits their preferred outcome.
In summary, we found that daily antibiotic use is the most effective strategy for prevention of recurrent UTI. Daily cranberry pills, daily estrogen therapy, and monthly acupuncture sessions were also effective at reducing UTI rate. Symptomatic self-treatment was the most favorable strategy in term of cost per QALY gained. Our findings provide clinically meaningful data to guide the physician–patient partnership with determining a preferred method of prevention for this common clinical problem.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Vanessa Vongkulluksn for her assistance with the data programming.
Financial support. This work was supported in part by the National Institutes of Health (grant number K23AI0183 to L. G. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–15. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 2.Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281–7. doi: 10.1097/01.ju.0000155596.98780.82. [DOI] [PubMed] [Google Scholar]
- 3.Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2006(Oct):1–209. [PubMed] [Google Scholar]
- 4.National Kidney and Urologic Diseases Information Clearinghouse. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2010. Kidney and urologic diseases statistics for the United States. [Google Scholar]
- 5.Foxman B, Frerichs RR. Epidemiology of urinary tract infection: I. Diaphragm use and sexual intercourse. Am J Public Health. 1985;75:1308–13. doi: 10.2105/ajph.75.11.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–3. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793–806. [PubMed] [Google Scholar]
- 8.Pfau A, Sacks T, Engelstein D. Recurrent urinary tract infections in premenopausal women: prophylaxis based on an understanding of the pathogenesis. J Urol. 1983;129:1153–7. doi: 10.1016/s0022-5347(17)52617-8. [DOI] [PubMed] [Google Scholar]
- 9.Walker EB, Barney DP, Mickelsen JN, Walton RJ, Mickelsen RA., Jr Cranberry concentrate: UTI prophylaxis. J Fam Pract. 1997;45:167–8. [PubMed] [Google Scholar]
- 10.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–62. [PubMed] [Google Scholar]
- 11.Stamm WE, Counts GW, Wagner KF, et al. Antimicrobial prophylaxis of recurrent urinary tract infections: a double-blind, placebo-controlled trial. Ann Intern Med. 1980;92:770–5. doi: 10.7326/0003-4819-92-6-770. [DOI] [PubMed] [Google Scholar]
- 12.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–6. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 13.Raz R, Colodner R, Rohana Y, et al. Effectiveness of estriol-containing vaginal pessaries and nitrofurantoin macrocrystal therapy in the prevention of recurrent urinary tract infection in postmenopausal women. Clin Infect Dis. 2003;36:1362–8. doi: 10.1086/374341. [DOI] [PubMed] [Google Scholar]
- 14.Privette M, Cade R, Peterson J, Mars D. Prevention of recurrent urinary tract infections in postmenopausal women. Nephron. 1988;50:24–7. doi: 10.1159/000185111. [DOI] [PubMed] [Google Scholar]
- 15.Pinggera GM, Feuchtner G, Frauscher F, et al. Effects of local estrogen therapy on recurrent urinary tract infections in young females under oral contraceptives. Eur Urol. 2005;47:243–9. doi: 10.1016/j.eururo.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.McMurdo ME, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63:389–95. doi: 10.1093/jac/dkn489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072–9. doi: 10.1016/s0002-9378(99)70597-1. [DOI] [PubMed] [Google Scholar]
- 19.Brumfitt W, Smith GW, Hamilton-Miller JM, Gargan RA. A clinical comparison between Macrodantin and trimethoprim for prophylaxis in women with recurrent urinary infections. J Antimicrob Chemother. 1985;16:111–20. doi: 10.1093/jac/16.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Brumfitt W, Hamilton-Miller JM, Walker S, Roberts D. Cefaclor as a prophylactic agent for recurrent urinary infections: a comparative trial with macrocrystalline nitrofurantoin. Drugs Exp Clin Res. 1992;18:239–44. [PubMed] [Google Scholar]
- 21.Brumfitt W, Hamilton-Miller JM, Smith GW, al-Wali W. Comparative trial of norfloxacin and macrocrystalline nitrofurantoin (Macrodantin) in the prophylaxis of recurrent urinary tract infection in women. Q J Med. 1991;81:811–20. [PubMed] [Google Scholar]
- 22.Brumfitt W, Hamilton-Miller JM. A comparative trial of low dose cefaclor and macrocrystalline nitrofurantoin in the prevention of recurrent urinary tract infection. Infection. 1995;23:98–102. doi: 10.1007/BF01833874. [DOI] [PubMed] [Google Scholar]
- 23.Brumfitt W, Cooper J, Hamilton-Miller JM. Prevention of recurrent urinary infections in women: a comparative trial between nitrofurantoin and methenamine hippurate. J Urol. 1981;126:71–4. doi: 10.1016/s0022-5347(17)54386-4. [DOI] [PubMed] [Google Scholar]
- 24.Aune A, Alraek T, LiHua H, Baerheim A. Acupuncture in the prophylaxis of recurrent lower urinary tract infection in adult women. Scand J Prim Health Care. 1998;16:37–9. doi: 10.1080/028134398750003386. [DOI] [PubMed] [Google Scholar]
- 25.Alraek T, Soedal LI, Fagerheim SU, Digranes A, Baerheim A. Acupuncture treatment in the prevention of uncomplicated recurrent lower urinary tract infections in adult women. Am J Public Health. 2002;92:1609–11. doi: 10.2105/ajph.92.10.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer AJ, Stuppy BA. Efficacy and safety of self-start therapy in women with recurrent urinary tract infections. J Urol. 1999;161:207–11. [PubMed] [Google Scholar]
- 28.Wong ES, McKevitt M, Running K, Counts GW, Turck M, Stamm WE. Management of recurrent urinary tract infections with patient-administered single-dose therapy. Ann Intern Med. 1985;102:302–7. doi: 10.7326/0003-4819-102-3-302. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence. Measuring effectiveness and cost effectiveness: the QALY. Available at: http://www.nice.org.uk/newsroom/features/measuringeffectivenessandcosteffectivenessthmeasu.jsp . Accessed 5 November 2010.
- 30.Le TP, Miller LG. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: a decision and cost analysis. Clin Infect Dis. 2001;33:615–21. doi: 10.1086/322603. [DOI] [PubMed] [Google Scholar]
- 31.Perfetto EM, Gondek K. Escherichia coli resistance in uncomplicated urinary tract infection: a model for determining when to change first-line empirical antibiotic choice. Manag Care Interface. 2002;15:35–42. [PubMed] [Google Scholar]
- 32.McKinnell JA, Stollenwerk NS, Jung CW, Miller LG. Nitrofurantoin compares favorably to recommended agents as empirical treatment of uncomplicated urinary tract infections in a decision and cost analysis. Mayo Clinic proceedings. Mayo Clinic. 2011;86:480–8. doi: 10.4065/mcp.2010.0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg M. Pharmacoeconomics of treating uncomplicated urinary tract infections. Int J Antimicrob Agents. 1999;11:247–51. doi: 10.1016/s0924-8579(99)00024-2. ; discussion 261–4. [DOI] [PubMed] [Google Scholar]
- 34.Barry HC, Ebell MH, Hickner J. Evaluation of suspected urinary tract infection in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract. 1997;44:49–60. [PubMed] [Google Scholar]
- 35.Eron LJ, Passos S. Early discharge of infected patients through appropriate antibiotic use. Arch Intern Med. 2001;161:61–5. doi: 10.1001/archinte.161.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Benoit G, Yataghene Y, Bensadoun H, et al. Acute pyelonephritis in women. Short-term hospitalization [in French] Presse Med. 1993;22:1724–8. [PubMed] [Google Scholar]
- 37.Bailey R, Lynn K, Robson R, Peddie B, Smith A. Comparison of ciprofloxacin and netilmicin for the treatment of acute pyelonephritis. N Z Med J. 1992;105:102–3. [PubMed] [Google Scholar]
- 38.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–58. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 39.Stamm WE, McKevitt M, Roberts PL, White NJ. Natural history of recurrent urinary tract infections in women. Rev Infect Dis. 1991;13:77–84. doi: 10.1093/clinids/13.1.77. [DOI] [PubMed] [Google Scholar]
- 40.Flach SD, Longenecker JC, Tape TG, Bryan TJ, Parenti C, Wigton RS. The relationship between treatment objectives and practice patterns in the management of urinary tract infections. Med Decis Making. 2003;23:131–9. doi: 10.1177/0272989X03251242. [DOI] [PubMed] [Google Scholar]
- 41.Dans PE, Klaus B. Dysuria in women. Johns Hopkins Med J. 1976;138:13–18. [PubMed] [Google Scholar]
- 42.Komaroff AL, Pass TM, McCue JD, Cohen AB, Hendricks TM, Friedland G. Management strategies for urinary and vaginal infections. Arch Intern Med. 1978;138:1069–73. [PubMed] [Google Scholar]
- 43.Echols RM, Tosiello RL, Haverstock DC, Tice AD. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin Infect Dis. 1999;29:113–9. doi: 10.1086/520138. [DOI] [PubMed] [Google Scholar]
- 44.Cunha BA. Intravenous-to-oral antibiotic switch therapy. A cost-effective approach. Postgrad Med. 1997;101 doi: 10.3810/pgm.1997.04.199. 111–2, 115–8, 122–3 passim. [DOI] [PubMed] [Google Scholar]
- 45.Henry NK, Schultz HJ, Grubbs NC, Muller SM, Ilstrup DM, Wilson WR. Comparison of ciprofloxacin and co-trimoxazole in the treatment of uncomplicated urinary tract infection in women. J Antimicrob Chemother. 1986;18(suppl D):103–6. doi: 10.1093/jac/18.supplement_d.103. [DOI] [PubMed] [Google Scholar]
- 46.Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother. 1999;43(suppl A):67–75. [PubMed] [Google Scholar]
- 47.Grubbs NC, Schultz HJ, Henry NK, Ilstrup DM, Muller SM, Wilson WR. Ciprofloxacin versus trimethoprim-sulfamethoxazole: treatment of community-acquired urinary tract infections in a prospective, controlled, double-blind comparison. Mayo Clin Proc. 1992;67:1163–8. doi: 10.1016/s0025-6196(12)61146-x. [DOI] [PubMed] [Google Scholar]
- 48.Malinverni R, Glauser MP. Comparative studies of fluoroquinolones in the treatment of urinary tract infections. Rev Infect Dis. 1988;10(suppl 1):S153–63. doi: 10.1093/clinids/10.supplement_1.s153. [DOI] [PubMed] [Google Scholar]
- 49.American Hospital Association. Hospital statistics 2007 edition. Chicago: American Hospital Association; 2007. [Google Scholar]
- 50.Centers for Medicare and Medicaid Services. Physician fee schedule look-up tool. Available at: http://www.cms.hhs.gov/PFSlookup/02_PFSSearch.asp-TopOfPage. Accessed 17 March 2008.
- 51.Neuner J, Hamel M, Phillips R, Bona K, Aronson M. Diagnosis and management of adults with pharyngitis. A cost-effectiveness analysis. Ann Intern Med. 2003;139:113–22. doi: 10.7326/0003-4819-139-2-200307150-00011. [DOI] [PubMed] [Google Scholar]
- 52.Rubin N, Foxman B. The cost-effectiveness of placing urinary tract infection treatment over the counter. J Clin Epidemiol. 1996;49:1315–21. doi: 10.1016/s0895-4356(96)00218-1. [DOI] [PubMed] [Google Scholar]
- 53.Sobel J, Wiesenfeld H, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidasis. N Engl J Med. 2004;351:876–83. doi: 10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37:85–95. doi: 10.1016/0021-9681(84)90050-x. [DOI] [PubMed] [Google Scholar]
- 55.Talan D, Stamm W, Hooton T, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA. 2000;283:1583–90. doi: 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 56.McCarty J, Richard G, Huck W, et al. A randomized trial of short-course ciprofloxacin, ofloxacin, or trimethoprim/sulfamethoxazole for the treatment of acute urinary tract infection in women. Am J Med. 1999;106:292–9. doi: 10.1016/s0002-9343(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 57.Henry D, Bettis R, Riffer E, et al. Comparison of once-daily extended-release ciprofloxacin and conventional twice-daily ciprofloxacin for the treatment of uncomplicated urinary tract infection in women. Clin Ther. 2002;24:2088–103. doi: 10.1016/s0149-2918(02)80099-6. [DOI] [PubMed] [Google Scholar]
- 58.Hooton T, Winter C, Tiu F, Stamm W. Randomized comparative trial and cost analysis of 3-day antimicrobial regimens for treatment of acute cystitis in women. JAMA. 1995;273:41–5. [PubMed] [Google Scholar]
- 59.Spencer R, Moseley D, Greensmith M. Nitrofurantoin modified release versus trimethoprim or co-trimoxazole in the treatment of uncomplicated urinary tract infection in general practice. J Antimicrob Chemother. 1994;33:121–9. doi: 10.1093/jac/33.suppl_a.121. [DOI] [PubMed] [Google Scholar]
- 60.Stein G. Comparison of single-dose fosfomycin and a 7-day course of nitrofurantoin in female patients with uncomplicated urinary tract infection. Clin Ther. 1999;21:1864–72. doi: 10.1016/S0149-2918(00)86734-X. [DOI] [PubMed] [Google Scholar]
- 61.2010 Red Book. 114th ed. Montvale, NJ: Medical Economics Company; 2010. [Google Scholar]
- 62.Takahashi S, Hirose T, Satoh T, et al. Efficacy of a 14-day course of oral ciprofloxacin therapy for acute uncomplicated pyelonephritis. J Infect Chemother. 2001;7:255–57. doi: 10.1007/s101560170023. [DOI] [PubMed] [Google Scholar]
- 63.Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51:738–45. doi: 10.1002/mnfr.200600275. [DOI] [PubMed] [Google Scholar]
- 64.Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52:23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87:143–50. doi: 10.1016/j.mayocp.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beerepoot MA, ter Riet G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind noninferiority trial in premenopausal women. Arch Intern Med. 2011;171:1270–8. doi: 10.1001/archinternmed.2011.306. [DOI] [PubMed] [Google Scholar]
- 67.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005131.pub2. CD005131. [DOI] [PubMed] [Google Scholar]
- 68.Cardozo L, Lose G, McClish D, Versi E, de Koning Gans H. A systematic review of estrogens for recurrent urinary tract infections: third report of the hormones and urogenital therapy (HUT) committee. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:15–20. doi: 10.1007/s001920170088. [DOI] [PubMed] [Google Scholar]
- 69.Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scand J Prim Health Care. 1994;12:239–43. doi: 10.3109/02813439409029247. [DOI] [PubMed] [Google Scholar]
- 70.Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704–12. doi: 10.1001/archinternmed.2012.777. [DOI] [PubMed] [Google Scholar]