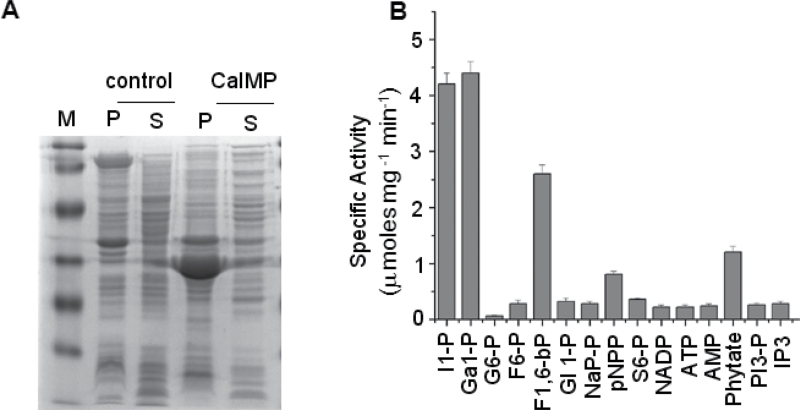
Fig. 3.
Biochemical characterization of CaIMP. (A) 12% SDS PAGE analysis of CaIMP over-expressed protein in E. coli BL21. [Control, pET23d empty vector transformed induced cells; CaIMP, CaIMP transformed induced cells; M, molecular weight marker; P, pellet fractions; S, soluble fractions.] (B) Substrate specificity of purified recombinant CaIMP protein. Substrate specificity was determined using all the substrates at a final concentration of 30 μM and at 37 °C. [I1-P, d-myo-inositol 1-phosphate; Ga1-P, l-galactose 1-phosphate; G6-P, α-d-glucose 6-phosphate; F6-P, d-fructose 6-phosphate; F1,6-bP, d-fructose 1,6-bisphosphate; Gl1-P, β-glycerol 1-phosphate; NaP-P, sodium pyrophosphate; pNPP, p-nitrophenyl phosphate; S6-P, d-sorbitol 6-phosphate; NADP, β-nicotinamide adenine dinucleotide phosphate; ATP, adenosine triphosphate; AMP, adenosine monophosphate; PI3-P, phosphotidyl inositol triphosphate; IP3, inositol triphosphate.] In each case values are mean ±SE of three replicates. In each experiment, 10 μg purified protein was used. Specific activity was calculated as μmol Pi released mg–1 protein min–1.