Abstract
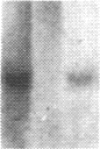
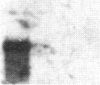
The carbonic anhydrases (CA) are a class of metalloenzymes that catalyze the reversible hydration of carbon dioxide. The genes for the carbonic anhydrase isozymes are members of a multigene family that are differentially expressed in a number of cell types. We have isolated a full-length representative of a CAIII mRNA transcript from an adult human muscle cDNA library, and we present the complete nucleotide sequence of this cDNA clone. RNA blots demonstrate that CAIII messages can be detected in a variety of cell types but that high-level expression is limited to human fetal and adult skeletal muscle and to rodent slow skeletal muscle and liver. In addition, we have used a panel of human-mouse cell hybrids to localize the human CAIII gene to chromosome 8. Previous reports have established the CAI and CAII isozyme genes to be closely linked on chromosome 8, and the assignment of the CAIII gene to the same chromosome raises the possibility that these genes may all be linked at a single complex locus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton P. J., Cohen A., Robert B., Fiszman M. Y., Bonhomme F., Guénet J. L., Leader D. P., Buckingham M. E. The myosin alkali light chains of mouse ventricular and slow skeletal muscle are indistinguishable and are encoded by the same gene. J Biol Chem. 1985 Jul 15;260(14):8578–8584. [PubMed] [Google Scholar]
- Boyer S. H., Ostrer H., Smith K. D., Young K. E., Noyes A. N. Isolation of cDNA clones for rabbit red cell carbonic anhydrase and catalase: a pilot study directed at isolation of coordinately expressed genes. Ann N Y Acad Sci. 1984;429:324–331. doi: 10.1111/j.1749-6632.1984.tb12356.x. [DOI] [PubMed] [Google Scholar]
- Carter N. D. Carbonic anhydrase isozymes in Cavia porcellus, Cavia aperea and their hybrids. Comp Biochem Physiol B. 1972 Nov 15;43(3):743–747. doi: 10.1016/0305-0491(72)90159-9. [DOI] [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Withers E., Demuth D., Watt R., Venta P. J., Tashian R. E. The nucleotide sequence and derived amino acid sequence of cDNA coding for mouse carbonic anhydrase II. Gene. 1983 Nov;25(2-3):325–332. doi: 10.1016/0378-1119(83)90237-8. [DOI] [PubMed] [Google Scholar]
- DeSimone J., Linde M., Tashian R. E. Evidence for linkage of carbonic anhydrase isozyme genes in the pig-tailed macaque, Macaca nemestrina. Nat New Biol. 1973 Mar 14;242(115):55–56. doi: 10.1038/newbio242055a0. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Barlow J. H., Konialis C. P., Povey S., Butterworth P. H. Assignment of the gene determining human carbonic anhydrase, CAI, to chromosome 8. Ann Hum Genet. 1986 May;50(Pt 2):123–129. doi: 10.1111/j.1469-1809.1986.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Stern R. H., Womack J. E., Davisson M. T., Roderick T. H., Reynolds S. C. Evolution of mammalian carbonic anhydrase loci by tanden duplication: close linkage of Car-1 and Car-2 to the centromere region of chromosome 3 of the mouse. Biochem Genet. 1976 Aug;14(7-8):651–660. doi: 10.1007/BF00485843. [DOI] [PubMed] [Google Scholar]
- Engberg P., Millqvist E., Pohl G., Lindskog S. Purification and some properties of carbonic anhydrase from bovine skeletal muscle. Arch Biochem Biophys. 1985 Sep;241(2):628–638. doi: 10.1016/0003-9861(85)90589-2. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Hardeman E., Wade R., Kedes L., Gunning P. Isolation of full-length cDNAs encoding abundant adult human skeletal muscle mRNAs. Gene. 1985;38(1-3):177–188. doi: 10.1016/0378-1119(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett-Emmett D., Hopkins P. J., Tashian R. E., Czelusniak J. Origins and molecular evolution of the carbonic anhydrase isozymes. Ann N Y Acad Sci. 1984;429:338–358. doi: 10.1111/j.1749-6632.1984.tb12359.x. [DOI] [PubMed] [Google Scholar]
- Hewett-Emmett D., Welty R. J., Tashian R. E. A widespread silent polymorphism of human carbonic anhydrase III (31 Ile in equilibrium Val): implications for evolutionary genetics. Genetics. 1983 Oct;105(2):409–420. doi: 10.1093/genetics/105.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery S., Edwards Y., Carter N. Distribution of CAIII in fetal and adult human tissue. Biochem Genet. 1980 Oct;18(9-10):843–849. doi: 10.1007/BF00500117. [DOI] [PubMed] [Google Scholar]
- Jeffery S., Wilson C. A., Mode A., Gustafsson J. A., Carter N. D. Effects of hypophysectomy and growth hormone infusion on rat hepatic carbonic anhydrases. J Endocrinol. 1986 Jul;110(1):123–126. doi: 10.1677/joe.0.1100123. [DOI] [PubMed] [Google Scholar]
- Kendall A. G., Tashian R. E. Erythrocyte carbonic anhydrase I: inherited deficiency in humans. Science. 1977 Jul 29;197(4302):471–472. doi: 10.1126/science.406674. [DOI] [PubMed] [Google Scholar]
- Konialis C. P., Barlow J. H., Butterworth P. H. Cloned cDNA for rabbit erythrocyte carbonic anhydrase I: A novel erythrocyte-specific probe to study development in erythroid tissues. Proc Natl Acad Sci U S A. 1985 Feb;82(3):663–667. doi: 10.1073/pnas.82.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. C., Isenberg H., Hopkinson D. A., Edwards Y. H. Isolation of a cDNA clone for the human muscle specific carbonic anhydrase, CAIII. Ann Hum Genet. 1985 Jul;49(Pt 3):241–251. doi: 10.1111/j.1469-1809.1985.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J., McMillan S., Hopkinson D., Edwards Y. H. Nucleotide sequence and derived amino acid sequence of a cDNA encoding human muscle carbonic anhydrase. Gene. 1986;41(2-3):233–239. doi: 10.1016/0378-1119(86)90103-4. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sakaguchi A. Y., Shows T. B. Coronavirus 229E susceptibility in man-mouse hybrids is located on human chromosome 15. Somatic Cell Genet. 1982 Jan;8(1):83–94. doi: 10.1007/BF01538652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Jeffery S., Wilson C., Carter N. Radioimmunoassay of carbonic anhydrase III in rat tissues. Biochem J. 1984 Mar 1;218(2):281–284. doi: 10.1042/bj2180281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T., Eddy R., Haley L., Byers M., Henry M., Fujita T., Matsui H., Taniguchi T. Interleukin 2 (IL2) is assigned to human chromosome 4. Somat Cell Mol Genet. 1984 May;10(3):315–318. doi: 10.1007/BF01535253. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983 May;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venta P. J., Shows T. B., Curtis P. J., Tashian R. E. Polymorphic gene for human carbonic anhydrase II: a molecular disease marker located on chromosome 8. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4437–4440. doi: 10.1073/pnas.80.14.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. H., Silverman D. N. Carbonic anhydrase activity in mitochondria from rat liver. J Biol Chem. 1982 Jun 25;257(12):6850–6855. [PubMed] [Google Scholar]
- Wendorff K. M., Nishita T., Jabusch J. R., Deutsch H. F. The sequence of equine muscle carbonic anhydrase. J Biol Chem. 1985 May 25;260(10):6129–6132. [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]