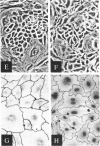
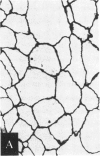
Abstract
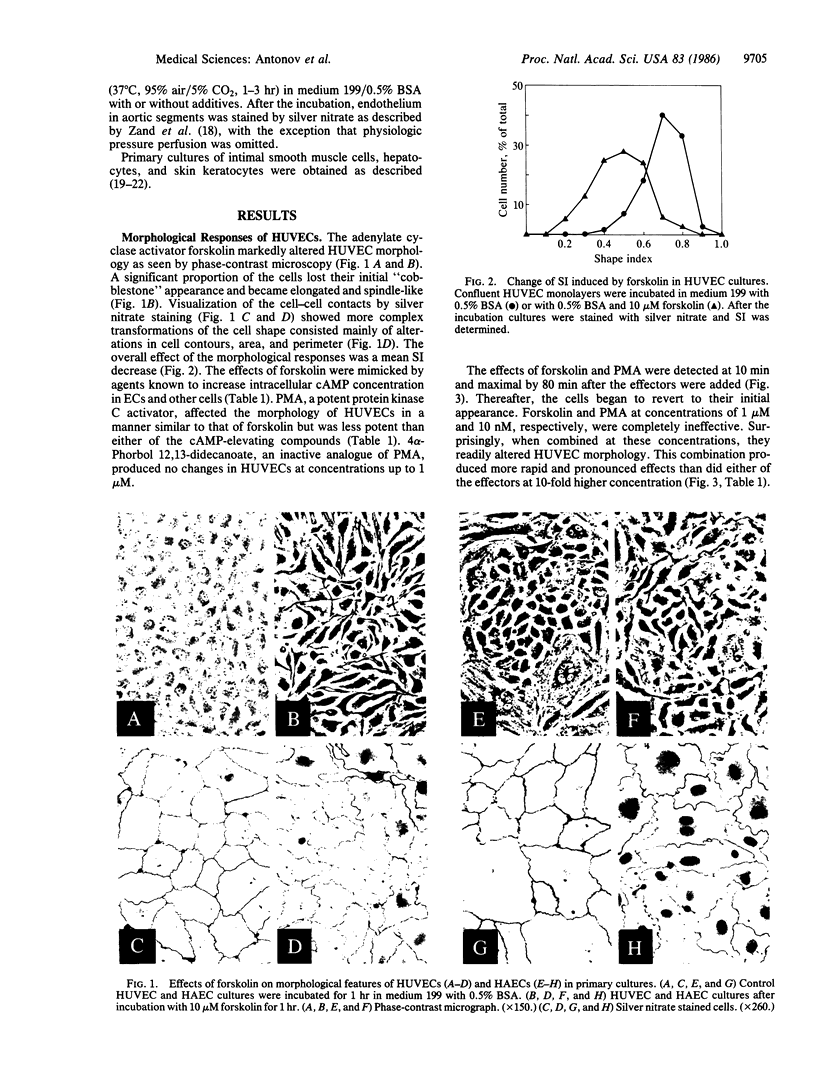
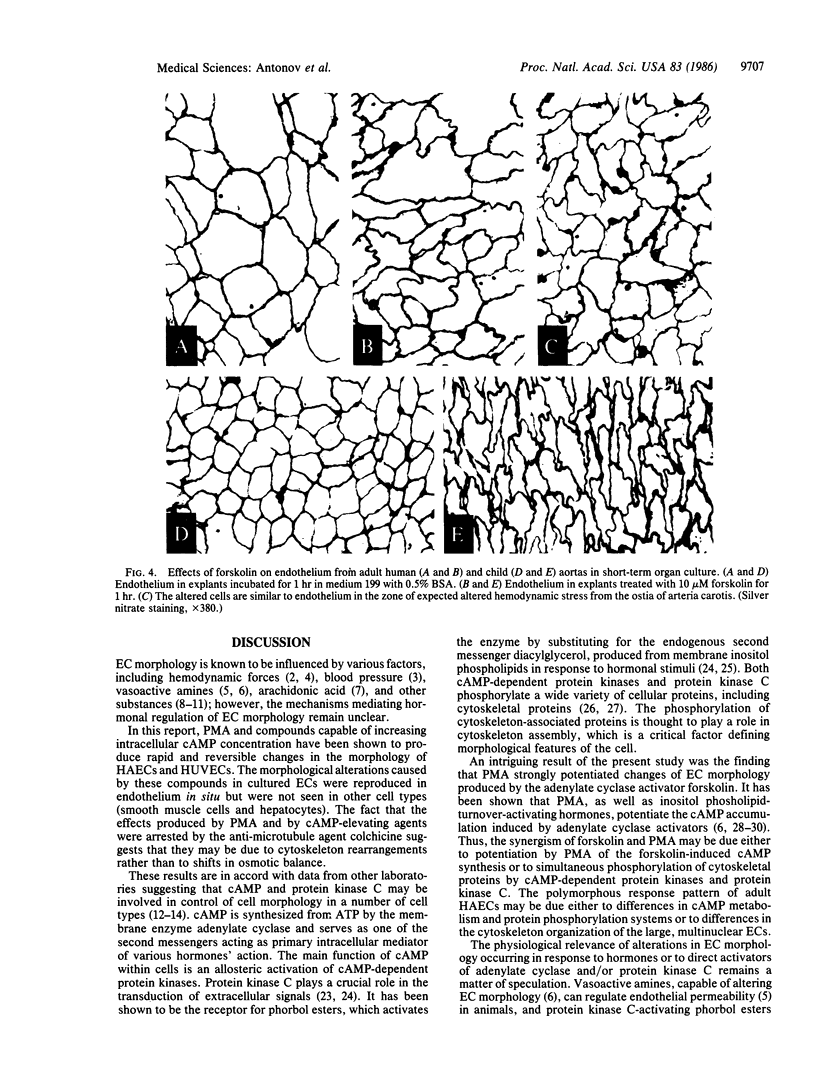
The morphological effects on human endothelial cells of phorbol 12-myristate 13-acetate (PMA) and of agents that increase intracellular cAMP concentration were studied. The adenylate cyclase activator forskolin (10 microM), the cyclic nucleotide phosphodiesterase inhibitor methylisobutylxanthine (100 microM), dibutyryl-cAMP (10 microM), histamine (10 microM), and PMA (0.1 microM) significantly altered the morphology of human aortic and umbilical vein endothelial cells in primary cultures. These effects reached a maximum 40-80 min after the effector addition and became negligible 30-60 min after its removal. PMA and forskolin were strongly synergistic in altering endothelial cell morphology. All the effects of cAMP-elevating compounds and of PMA were abolished completely by 1 microM colchicine. In explants taken from human adult or child aortas, forskolin and PMA produced alterations in endothelial morphology qualitatively identical to those observed in endothelial cell cultures. Endothelium in these preparations closely resembled that found in zones of expected altered hemodynamic stresses of human aorta. Our data suggest that the morphology of endothelium in vivo may be regulated by separate or synergistic action of hormone-dependent adenylate cyclase and of inositol phospholipid turnover systems and might be important for maintenance of endothelial monolayer integrity under normal physiological and pathological conditions.
Full text
PDF
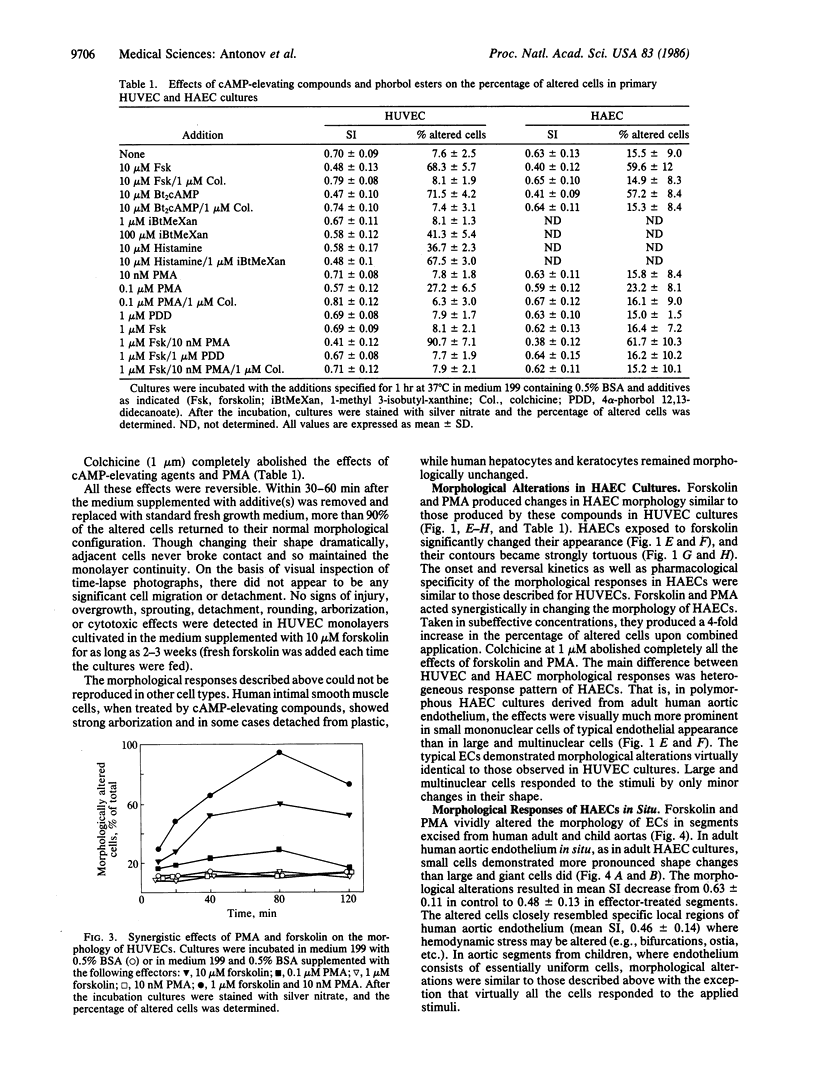

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Antonov A. S., Nikolaeva M. A., Klueva T. S., Romanov YuA, Babaev V. R., Bystrevskaya V. B., Perov N. A., Repin V. S., Smirnov V. N. Primary culture of endothelial cells from atherosclerotic human aorta. Part 1. Identification, morphological and ultrastructural characteristics of two endothelial cell subpopulations. Atherosclerosis. 1986 Jan;59(1):1–19. doi: 10.1016/0021-9150(86)90027-4. [DOI] [PubMed] [Google Scholar]
- Babaev V. R., Antonov A. S., Romanov Iu A., Rukosuev V. S., Repin V. S. Raspredelenie miozina v pervichnoi kul'ture kletok intimy aorty cheloveka. Biull Eksp Biol Med. 1984 Mar;97(3):350–352. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- De Chastonay C., Gabbiani G., Elemér G., Hüttner I. Remodeling of the rat aortic endothelial layer during experimental hypertension. Changes in replication rate, cell density, and surface morphology. Lab Invest. 1983 Jan;48(1):45–52. [PubMed] [Google Scholar]
- Dedman J. R., Brinkley B. R., Means A. R. Regulation of microfilaments and microtubules by calcium and cyclic AMP. Adv Cyclic Nucleotide Res. 1979;11:131–174. [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A., Brosstad F. Effects of thrombin on the integrity of monolayers of cultured human endothelial cells. Thromb Res. 1982 Sep 1;27(5):575–584. doi: 10.1016/0049-3848(82)90304-8. [DOI] [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A. Effects of divalent cations and various vasoactive and haemostatically active agents on the integrity of monolayers of cultured human endothelial cells. Thromb Res. 1981 Feb 1;21(3):273–284. doi: 10.1016/0049-3848(81)90165-1. [DOI] [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A., Nilsen E. The effect of thrombin on fibronectin in cultured human endothelial cells. Thromb Res. 1985 Mar 1;37(5):583–593. doi: 10.1016/0049-3848(85)90091-x. [DOI] [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A., Nilsen E. Thrombin-induced shape changes of cultured endothelial cells: metabolic and functional observations. Thromb Res. 1983 Oct 1;32(1):57–66. doi: 10.1016/0049-3848(83)90154-8. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosykh V. A., Preobrazhensky S. N., Ivanov V. O., Tsibulsky V. P., Repin V. S., Smirnov V. N. High-affinity association and degradation of 125I-labelled low density lipoproteins by human hepatocytes in primary culture. FEBS Lett. 1985 Apr 8;183(1):17–20. doi: 10.1016/0014-5793(85)80944-3. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Adamson S. L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res. 1981 Apr;48(4):481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- Levesque M. J., Liepsch D., Moravec S., Nerem R. M. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986 Mar-Apr;6(2):220–229. doi: 10.1161/01.atv.6.2.220. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. The role of prostacyclin in vascular tissue. Fed Proc. 1979 Jan;38(1):66–71. [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Nabika T., Nara Y., Yamori Y., Lovenberg W., Endo J. Angiotensin II and phorbol ester enhance isoproterenol- and vasoactive intestinal peptide (VIP)-induced cyclic AMP accumulation in vascular smooth muscle cells. Biochem Biophys Res Commun. 1985 Aug 30;131(1):30–36. doi: 10.1016/0006-291x(85)91765-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Kosykh V. A., Repin V. S., Smirnov V. N. Cell proliferation in normal and atherosclerotic human aorta. I. Flow cytofluorometric determination of cellular deoxyribonucleic acid content. Lab Invest. 1983 Apr;48(4):395–398. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Sandra A., Bar R. S., Dolash S., Marshall S. J., Kaduce T. L., Spector A. A. Morphological alterations in cultured endothelial cells induced by arachidonic acid. Exp Cell Res. 1985 Jun;158(2):484–492. doi: 10.1016/0014-4827(85)90471-9. [DOI] [PubMed] [Google Scholar]
- Shepro D., Welles S. L., Hechtman H. B. Vasoactive agonists prevent erythrocyte extravasation in thrombocytopenic hamsters. Thromb Res. 1984 Aug 15;35(4):421–430. doi: 10.1016/0049-3848(84)90234-2. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Jeffs R. A., Daniel K., Nambi P., Lefkowitz R. J. Phorbol diester treatment promotes enhanced adenylate cyclase activity in frog erythrocytes. Arch Biochem Biophys. 1986 Jan;244(1):373–381. doi: 10.1016/0003-9861(86)90126-8. [DOI] [PubMed] [Google Scholar]
- Smith J. B. Beta-adrenergic stimulation inhibits calcium efflux and alters the morphology of cultured arterial muscle cells. J Cell Physiol. 1984 Nov;121(2):375–382. doi: 10.1002/jcp.1041210215. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Johnson D. D., Phan N. T., Wilcox R. Phorbol ester inhibits myoblast fusion and activates beta-adrenergic receptor coupled adenylate cyclase. FEBS Lett. 1985 Jul 8;186(2):281–285. doi: 10.1016/0014-5793(85)80725-0. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanecek J., Sugden D., Weller J., Klein D. C. Atypical synergistic alpha 1- and beta-adrenergic regulation of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in rat pinealocytes. Endocrinology. 1985 Jun;116(6):2167–2173. doi: 10.1210/endo-116-6-2167. [DOI] [PubMed] [Google Scholar]
- Welles S. L., Shepro D., Hechtman H. B. Vasoactive amines modulate actin cables (stress fibers) and surface area in cultured bovine endothelium. J Cell Physiol. 1985 Jun;123(3):337–342. doi: 10.1002/jcp.1041230307. [DOI] [PubMed] [Google Scholar]
- Zand T., Underwood J. M., Nunnari J. J., Majno G., Joris I. Endothelium and "silver lines". An electron microscopic study. Virchows Arch A Pathol Anat Histol. 1982;395(2):133–144. doi: 10.1007/BF00429607. [DOI] [PubMed] [Google Scholar]