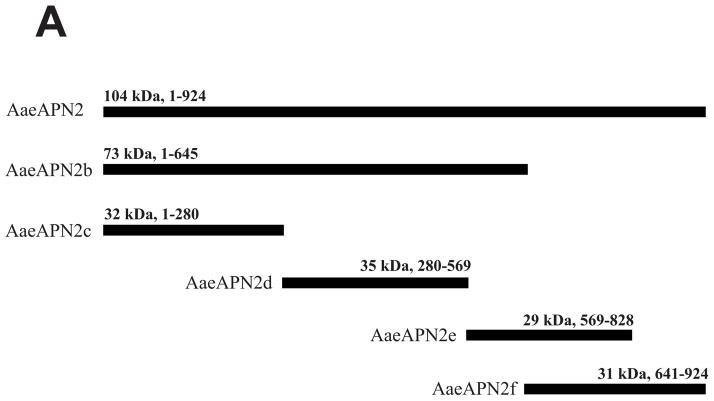
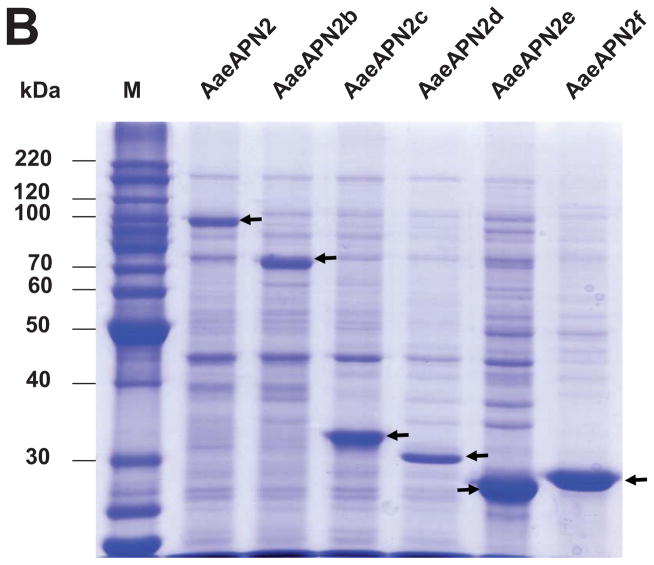
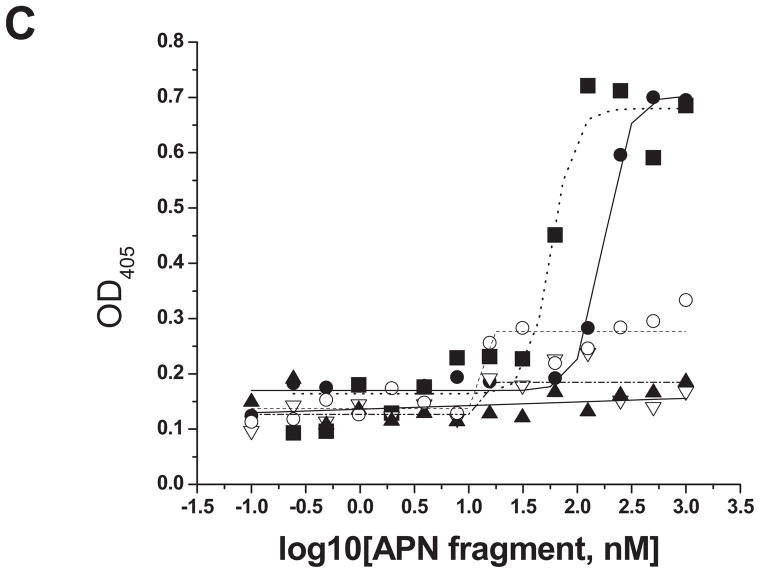
Figure 3. Cry11Aa binds AaeAPN2 and its partial fragments.
A. Schematic for isolation of partial AaeAPN2 fragments (b-f). The numbers above each bar refer to the molecular weight of the protein, and amino acid residues are that of the full-length AaeAPN2 protein. A Cry11Aa toxin binding site was mapped to aa 569–641. B. Expression of partial AaeAPN2 proteins. Five overlapping AaeAPN2 cDNA fragments were generated by PCR or by using existing restriction sites. These were then subcloned into pQE series expression vectors and expressed in E. coli M15(pREP4). The inclusion bodies of expressed proteins were obtained and separated by 12% SDS-PAGE gels. Arrows indicate expected size proteins. C. Cry11Aa binding to AaeAPN2 fragments. AaeAPN2a (■), AaeAPN2b (●) and AaeAPN2e (○) show higher binding to immobilized Cry11Aa toxin (0.4 μg) with increased APN protein levels. No binding was observed with AaeAPN2c (▽) and AaeAPN2d (▲).