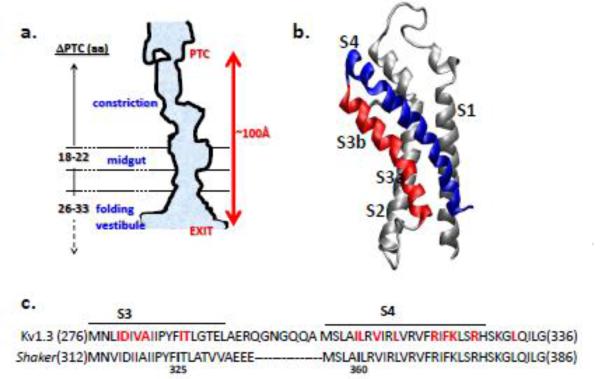
Figure 1. Biogenic participants.
(a) Cartoon of ribosome tunnel (after Wilson & Beckmann 201152). The constriction is a region of narrower dimensions approximately 25-30Å from the PTC; the midgut is a region that discriminates side chain volumes53; the folding vestibule begins at a tunnel location that is approximately 80Å from the PTC, near the exit port4. As indicated by the dashed arrow, its terminal border has not yet been defined. Numbers for ΔPTC are estimates derived from previous studies of modification of nascent chain cysteines in the tunnel4, 53. This angstrom distance is an estimate based on the observation that engineered cysteines located 26-33 residues from the PTC are accessible and that an extended peptide displaces 3-3.4 Å/amino acid. (b) Voltage-sensor domain (VSD) of a Kv channel. The S3 and S4 are red and blue, respectively. S1 and S2 are shown in gray. Model drawn in DS Viewer Pro using coordinates of the Kv1.2/2.1 crystal structure 20. (c) Comparison of the primary sequences of the S3-S4 hairpin from Kv1.3 and Shaker channels. The amino acid sequence is indicated by a single-letter code. Shaker residues 336-355 are indicated by dashes. Cysteine engineered in Kv1.3 for crosslinking experiments are bolded in red. In Shaker, residues I325 and I360 (bolded in black) are crosslinked in the closed state when substituted with cysteines25.