Figure 5.
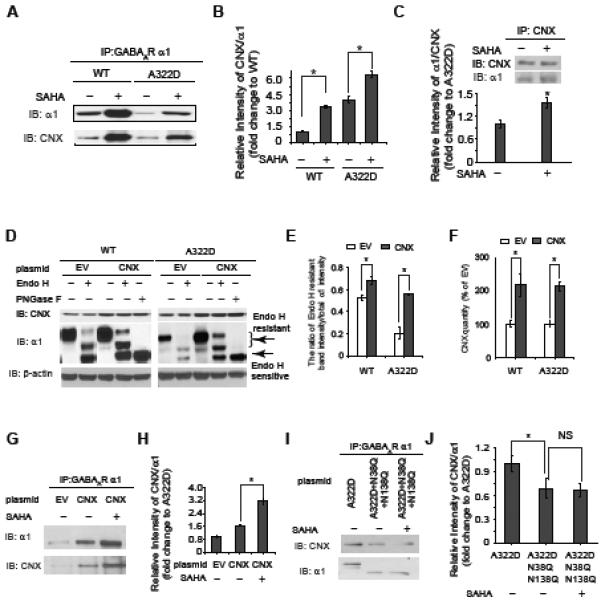
SAHA enhances the interaction between calnexin and the α1 subunit, and calnexin (CNX) promotes the α1 subunit folding in the ER.
(A and B) SAHA treatment (2.5 μM, 24 h) enhances the interaction between the α1 subunit and calnexin in HEK293 cells stably expressing WT α1β2γ2 or α1(A322D)β2γ2 GABAa receptors. Quantification of the relative intensity of calnexin/ α1 is shown in (B).
(C) Reverse immunoprecipitation confirms that SAHA treatment (2.5 μM, 24 h) enhances the interaction between the α1(A322D) subunit and calnexin.
(D, E and F) CNX overexpression increases endo H-resistant post-ER glycoform of the α1 subunit (n = 3) (D). PNGase F treatment serves as a control for unglycosylated α1 subunit. Quantification of the ratio of endo H-resistant / total α1 subunit bands is shown in (E), and quantification of CNX overexpression is shown in (F). EV: empty vector.
(G and H) SAHA treatment (2.5 μM, 24 h) enhances the interaction between the α1(A322D) subunit and overexpressed calnexin in HEK293 cells expressing α1(A322D)β2γ2 GABAa receptors (G). Quantification of the blots is shown in (H).
(I and J) Calnexin interacts with the α1(A322D) subunit in a glycan-dependent way. The N38Q/N138Q mutations in the α1(A322D) subunit disrupt the interaction between the N-glycans in the α1(A322D) subunit and calnexin (I). SAHA treatment does not change the interaction between the N38Q/N138Q mutant α1(A322D) subunit and calnexin (I). Quantification of the blots is shown in (J). NS: not significant.
Each data point in (B), (C), (E), (F), (H) and (J) is reported as mean ± SEM. * p < 0.05 See also Figure S4.
